Effect of inflating process on surface morphology of microspheres
-
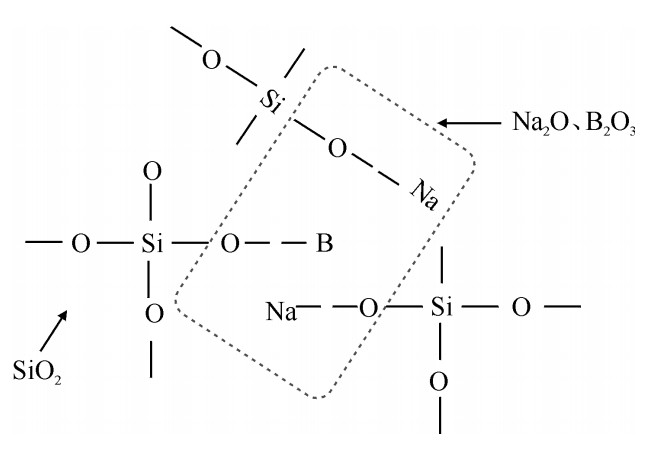
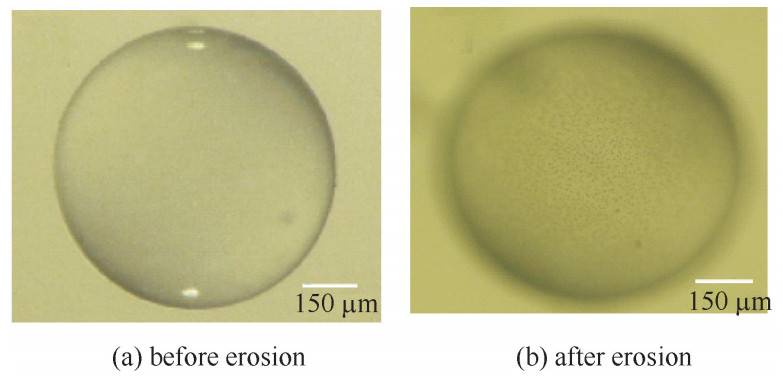
摘要: 对空心玻璃微球(HGM)在高温充气过程中的表面形貌进行了研究。分析局部晶化出现的时间和条件,并通过其表面形貌、成分分析探究了其随时间的变化规律。研究结果表明:影响HGM高温充气后表面形貌的因素主要有充气温度和充气压力;充气温度越高、压力越大表面形貌发生变化所需时间越短。导致表面形貌变化的本质原因是结晶分相,结晶成分主要由Na, B等元素组成。HGM的表面结晶分相使其表面粗糙度大幅度升高,但随着脱离充气环境后放置时间的延长而又逐渐降低。其表面粗糙度降低的主要原因是Na, B等元素的逆向迁移。Abstract: The morphology on the surface of hollow glass mircospheres(HGMs) in inflating process was investigated in this work. The experimental results indicate that the main factors influencing the surface of HGM include temperature and pressure, and higher temperature and pressure contributed to the formation of surface morphology. After the appearance of the erosion-like surface morphology, the surface roughness of HGMs increased significantly. However, after some time of storage, the surface roughness of HGMs decreased. The reason for erosion is crystallization and phase separation in essence. The components of crystal mainly consist of the elements of Na and B. The converse transfer of the elements of Na and B causes the decrease of surface roughness of HGMs.
-
Key words:
- hollow glass mircospheres /
- surface roughness /
- crystallization /
- phase separation
-
表 1 压力为3.5 MPa时不同温度下HGM出现浊化所需时间
Table 1. Time required to erode under same pressure and different temperature
T/℃ t/d T/℃ t/d 25 ≥150 300 ~4 100 ~80 400 ~2 200 ~20 表 2 温度为300 ℃时不同压力下HGM出现浊化所需时间
Table 2. Time required to erode under same temperature and different pressure
p/MPa t/d p/MPa t/d 0.1 ≥10 3.5 ~4 2 ~6 表 3 HGM表面粗糙度值
Table 3. Surface roughness value of HGM shells
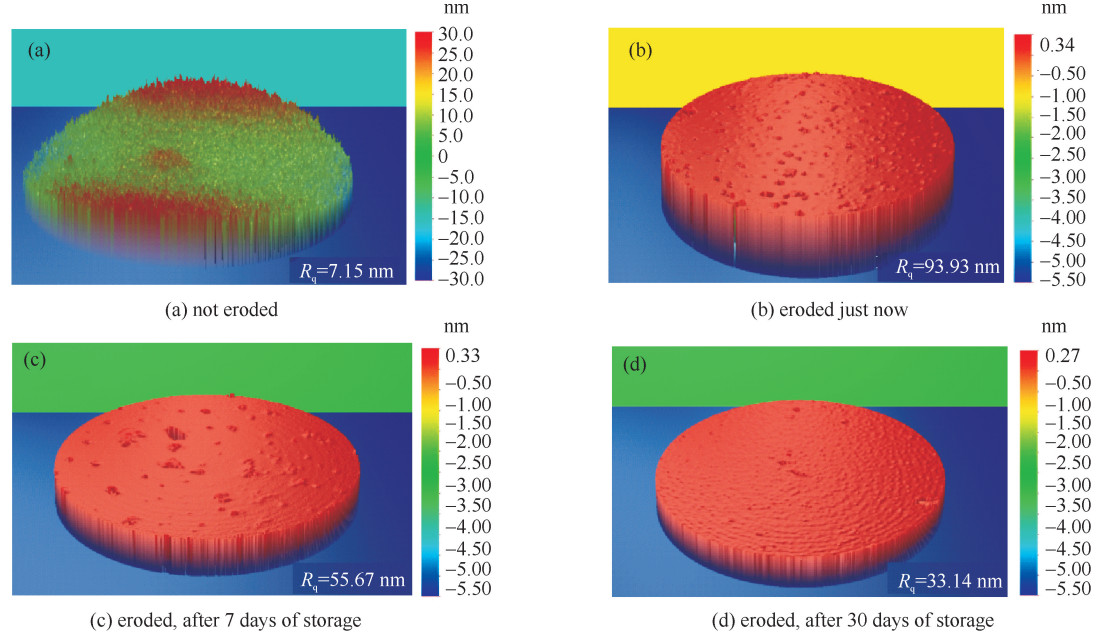
eroding condition Rq/nm not eroded 7.2 eroded just now(t=0) 93.9 eroded, after 7 days(t=7 d) 55.7 eroded, after 30 days(t=30 d) 35.1 表 4 结晶分相与未结晶分相HGM的基体元素质量百分比
Table 4. Mass fractions of elements in matrix of crystallizing and non-crystallized HGMs
element mass percentage/% original HGM crystallization begins(t=0) crystallizing for 7 d
(t=7 d)crystallizing for 30 d
(t=30 d)B 7.10 1.79 1.82 1.98 O 63.47 50.81 56.59 49.37 Na 1.03 2.99 4.12 4.10 Si 27.93 39.15 38.58 36.55 表 5 结晶分相与未结晶分相HGM的结晶区中元素质量百分比
Table 5. Mass fractions of element in crystalline zone of crystallizing HGMs
element mass percentage/% crystallization begins(t=0) crystallizing for 7 d (t=7 d) crystallizing for 30 d(t=30 d) B 44.06 43.69 7.75 O 4.25 30.51 47.44 Na 39.67 7.18 6.32 Si 3.85 18.24 31.92 -
[1] Craxton R S, Anderson K S, Boehly T R, et al. Direct-drive inertial confinement fusion: A review[J]. Phys Plasmas, 2015, 22: 110501. doi: 10.1063/1.4934714 [2] Nakai S, Takabe H. Principles of inertial confinement fusion—Physics of implosion and the concept of inertial fusion energy[J]. Rep Prog Phys. 1996, 59: 1071-1131. doi: 10.1088/0034-4885/59/9/002 [3] Belanger R P, Miller WJ. Glass shell preparation[J]. J Vac Sci Technol A, 1985, 3 (3): 1270-1273. doi: 10.1116/1.573082 [4] Qi Xiaobo, Gao Cong, Zhang Zhanwen, et al. Production and characterization of hollow glass microspheres with high diffusivity for hydrogen storage[J]. Hydrogen Energy, 2012(37): 1518-1530. [5] Deckman H W, Dunsmuir J H, Halpern G M. A drill, fill, and plug technique for fabricating laser fusion targets[J]. J Vac Sci Technol, 1981, 18 (3): 1258-1261. doi: 10.1116/1.570908 [6] Schmitt M L, Shelby J E, Hall M M. Preparation of hollow glass microspheres from sol-gel derived glass for application in hydrogen gas storage[J]. Journal of Non-Crystalline Solids, 2006, 352: 626-631. doi: 10.1016/j.jnoncrysol.2005.11.057 [7] Dalai S, Vijayalakshmi S, Sharma P, et al. Magnesium and iron loaded hollow glass microspheres(HGMs) for hydrogen storage[J]. Int Hydro Energy, 2014, 39: 16451-16458. doi: 10.1016/j.ijhydene.2014.03.062 [8] Nan J, John S. Electron irradiation induced phase decomposition in alkaline earth multi-component oxide glass[J]. J Applied Physics, 2002, 92: 2310-2316. doi: 10.1063/1.1496148 [9] 潘金龙. 玻璃工艺学[M]. 北京: 中国轻工业出版社, 1994.Pan Jinlong. Glass technology. Beijing: China Light Industry Press, 1994 [10] Qi X B, Gao C, Zhang Z W, et al. Fabrication and characterization of millimeter-sized glass shells for inertial confinement fusion targets[J]. Chem Eng Res Des, 2013, 91: 2497-508. doi: 10.1016/j.cherd.2013.03.010 [11] 邱龙会, 魏员, 傅依备, 等. 氘气通过高纵横比空心玻璃微球的实验研究[J]. 硅酸盐通报, 2001, 1: 3-5. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200101000.htmQiu Longhui, Wei Yun, Fu Yibei, et al. Experimental study on the permeability of D2 through hollow glass microspheres with high aspect ratios. Bulletin of the Chinese Ceramic Society, 2001, 1: 3-5 https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200101000.htm [12] 张占文, 李波, 唐永建, 等. ICF靶用空心玻璃微球耐压性能测试[J]. 强激光与粒子束, 2007, 19 (10): 1663-1666. http://www.hplpb.com.cn/article/id/3319Zhang Zhanwen, Li Bo, Tang Yongjian, et al. Measurement of mechanism properties of hollow glass microspheres used for ICF experiments. High Power Laser and Particle Beams, 2007, 19 (10): 1663-1666 http://www.hplpb.com.cn/article/id/3319 -





 下载:
下载: