Preparation and electrochemical performances of ternary LiNi1-x-yCoxMnyO2 cathode material
-
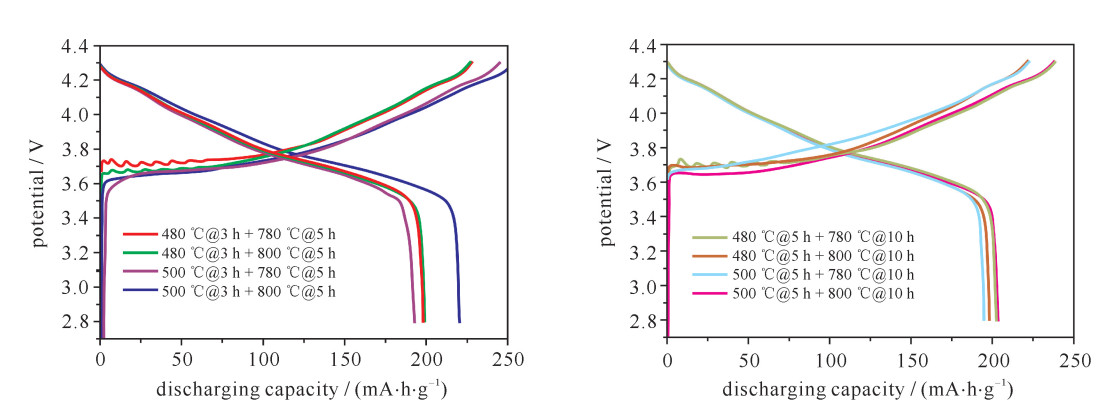
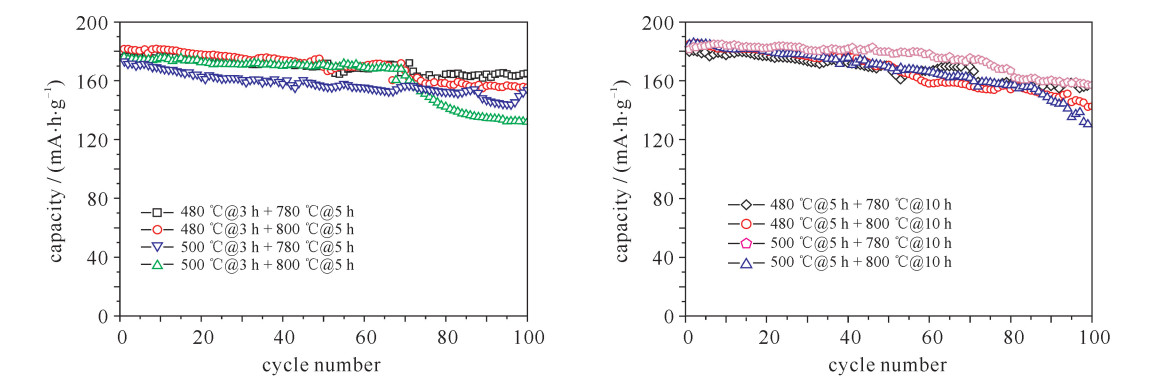
摘要: LiNi1-x-yCoxMnyO2正极材料作为最有商业化前途的锂离子电池正极材料,近年来成为研究者关注的焦点。但目前针对该材料合成工艺的研究还较少。对LiNi0.8Co0.15Mn0.05O2开展了不同的烧结工艺研究,并对制备出的正极材料进行了表征和性能测试。研究发现在0.1C (电池容量额定值)倍率下充放电比容量为200 mA·h·g-1左右,在1C倍率循环100次下,480 ℃@3 h + 780 ℃@5 h和500 ℃@5 h + 780 ℃@10 h两种烧结工艺下容量保持率分别为94%和86%,说明用这两种工艺制备的正极材料的综合性能最优。Abstract: As the most promising commercial lithium-ion battery, the LiNi1-x-yCoxMnyO2 cathode have become the focus of researchers in recent years. However, there are less researches on the synthetic process of this material. This paper discusses various sintering processes of LiNi0.8Co0.15Mn0.05O2, and the characterization and electrochemical performances testing of the prepared cathode materials. The research shows that the material has higher specific capacity (about 200 mA·h·g-1) at 0.1C (C is battery capacity rating value) charging-discharging rate. At 1C and 100 times of charging-discharging, the capacity retention ratios of 480 ℃@3 h + 780 ℃@5 h and 500 ℃@5 h + 780 ℃@10 h sintering processes are 94% and 86%, respectively, which illustrates the synthetical properties of the cathode materials prepared by these two processes are optimal.
-
目前,飞秒激光微纳米加工技术得到越来越广泛的应用。将双光子光聚合效应应用于微加工领域,显现出该技术较传统激光加工所不可比拟的独特优势,如可以突破光束衍射极限的限制,实现尺寸小于波长的亚微米甚至纳米级操作;能实现在材料内部三维空间上任意部位的超精细加工等[1]。2001年,Kawata等人采用飞秒激光双光子聚合的方法获得了一个10 μm长、7 μm高的“纳米牛”,首次从实验上突破了光学衍射极限,获得了120 nm的加工分辨率[2]。2005年,Takada等人通过在高分子树脂中添加阻聚剂获得了100 nm的聚合线条[3]。2007年,Tan等人利用双光子聚合的方法在两个支撑物结构之间获得了直径为30 nm的聚合物悬空线[4]。同年,董贤子等人采用高敏感的光引发剂,将双光子聚合加工的横向分辨率提高至80 nm[5]。2009年,Kawata等人对双光子聚合过程中影响分辨率的因素提出了新的机制[6]。2011年,赵震声等人在玻璃基板上获得了分辨率为35 nm和45 nm的聚合物纳米线条。2017年,林乐等人将径向偏振型飞秒脉冲激光引入双光子吸收模型来讨论分辨率更高的二维微纳加工[7]。本文从双光子光聚合过程中自由基浓度随时间变化出发,结合光镊效应对固化自由基分布范围的影响,对飞秒激光双光子加工分辨率进行了理论研究,理论结果与文献[1]和文献[5]中的实验数据基本符合。
1. 飞秒激光双光子过程的活性自由基浓度理论
双光子吸收是指在强光激发下,介质分子同时吸收两个光子,从基态跃迁到两倍光子能量的激发态的过程。单光子光聚合时光引发剂吸收一个紫外光子产生自由基,而双光子光聚合时光引发剂需要吸收两个红外光子产生自由基。在飞秒激光双光子加工过程中,当飞秒激光作用于高分子树脂样品时,其中的光引发剂吸收两个光子跃迁到激发态,之后转变为活性自由基。当样品中某处的活性自由基浓度达到临界的聚合浓度(聚合阈值ρth)时,就会引发聚合反应,产生固化的自由基[8]。在这个过程中,产生的活性自由基浓度ρ随时间的变化关系可表示为[5]
ρ=ϕσ2λ22(hc)2ρ0Npulse ∫I2dt (1) 式中:ϕ为自由基的转化效率;σ2为有效双光子吸收截面;λ为飞秒激光波长;h为普朗克常数;c为光速;ρ0为样品中光引发剂的初始浓度;Npulse为脉冲数; I为飞秒激光强度,表达式为[9]
I(r)=I0exp(−2r2ω02)exp(−4ln(2t2)τ2) (2) 式(2)中飞秒激光峰值强度为
I0=1τ×2Pπw20√4ln2π (3) 式中:τ为飞秒激光的脉冲宽度; P为激光功率; w0为束腰, 并且w0=2λ/(πNA),NA为数值孔径。
由于飞秒激光呈高斯分布,在中心位置较周围区域光强度更高,设光聚合时树脂样品的聚合阈值为ρth,如果某处的活性自由基浓度超过了聚合阈值,即ρ>ρth时,该处将引发自由基聚合反应,发生固化。自由基固化在宏观上则表现为样品中固化线条的产生,该线条在横向上的宽度便是加工得到的自由基线宽。在少量脉冲作用于树脂样品时,自由基浓度未达到聚合阈值,不会发生自由基固化,从而未产生固化自由基线宽;而当足够多的有效脉冲作用于该处时,活性自由基浓度一旦达到聚合阈值时,就会引发聚合。中心焦点处光强度最高,自由基浓度上升最快,最先达到自由基聚合阈值;随后焦点周围自由基浓度也逐渐达到自由基聚合阈值,从而焦点周围自由基固化区域由中心向外围逐渐扩大,表现为固化自由基线宽不断变大。
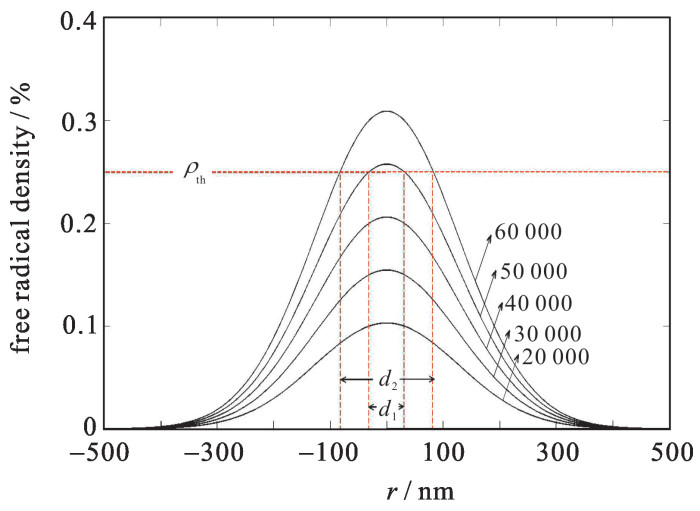
为了讨论方便,选用实验室常用的钛宝石飞秒激光器作为飞秒激光光源,其中λ=780 nm,τ=80 fs,重复频率fq=80 MHz, NA=1.42,h=6.62×10-34 J·s,c=3×108 m/s;选取ORMOCER材料为样品,其光引发剂的初始浓度ρ0为1%,光引发剂的聚合阈值为0.25%,ϕ=4×10-3,σ2=20×10-50 cm4·s[5]。根据式(1)~(3),图 1分析了自由基浓度的径向分布与脉冲次数的变化关系。从图 1可以看出,随着脉冲次数的增加,活性自由基浓度整体上升,当脉冲次数增加到48 000次左右时,激光中心处的活性自由基浓度达到聚合阈值,自由基开始固化产生线宽d。如图 1所示,经过50 000次脉冲作用之后线宽为d1, 60 000次脉冲作用之后线宽为d2。因此可以得知,作用于树脂样品的脉冲数越多,固化线宽越大。
自由基聚合所需临界脉冲数与激光功率、脉宽、重复频率等激光参数以及聚合阈值与初始自由基浓度的比值有关。考虑飞秒激光扫描树脂样品,这时与树脂样品作用的总脉冲数量可以看成曝光时间与飞秒激光重复频率的乘积,即Npulse=fqt。曝光时间又可以用激光束腰与扫描速度表示成t=2w0/vs, vs为扫描速度。下面考虑相邻两次脉冲作用下自由基浓度分布情况。由式(1)~(3)可知,第m次和第m+1次脉冲之后自由基浓度分布分别为
ρm=ϕσ2ρ04√π2ln2(λI(r)hc)2mτ (4) ρm+1=ϕσ2ρ04√π2ln2(λI(r)hc)2(m+1)τ (5) 在自由基聚合浓度阈值处,即ρm=ρm+1=ρth时,可以得到第m次和第m+1次脉冲时自由基浓度等于聚合浓度阈值时的半径关系
r2m+1−r2m=w204lnm+1m (6) 由上文中的分析我们知道, 样品中自由基浓度达到浓度阈值时自由基分布范围的半径就是自由基固化产生线宽的一半。由式(4)~(6)可以得到自由基固化区域半径表达式,从而得到飞秒激光双光子加工线宽表达式
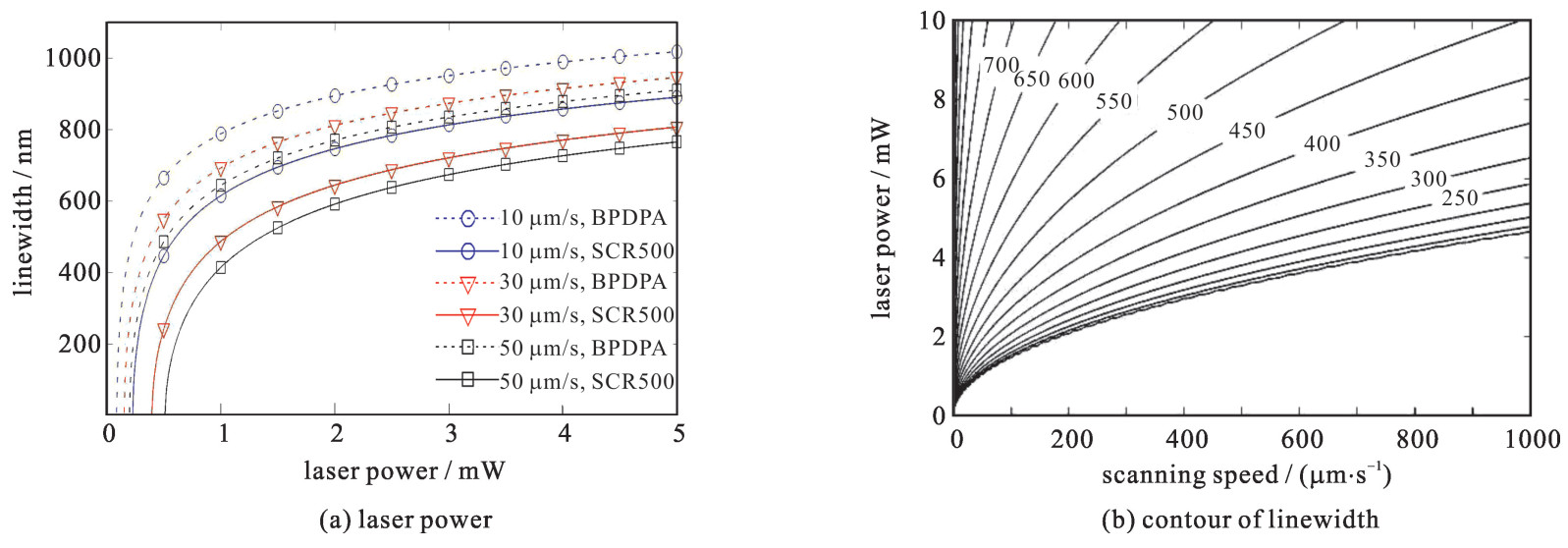
d=w0{ln[σ2ρ0ϕρth2w0vs(fqτ)√π8ln2(λPhc)2(1fqτ×1.88πw20)2]}1/2 (7) 为了比较不同扫描速度、激光功率以及不同光引发剂对于加工线宽的影响,我们选取了高敏感光引发剂材料BPDPA与传统光引发剂SCR500进行对比,根据式(7)可以得出图 2。图 2(a)中虚线为BPDPA材料,实线为SCR500材料。由图 2(a)可知,当激光功率不变时,扫描速度越大,线宽越小;当扫描速度一定时,激光功率越小,线宽越小;当扫描速度相同时,高敏感光引发剂引发自由基聚合所需的临界功率更小。图 2(b)为加工线宽随扫描速度与激光功率变化的等值线图。由图 2(b)可知,在加工过程中,每一个激光功率都对应有一个临界的扫描速度值,同样每一个扫描速度也都对应有一个临界的激光功率。因此,并不能获得任意小的加工线宽。
2. 飞秒激光双光子过程的光镊集聚效应
光镊(OT)理论指出,光与物质发生相互作用时彼此交换动量,使得受照物体获得一个力或力矩,从而使物体发生位移,导致速度或方位的变化。对于直径小于激光波长的颗粒,粒子小球在光束焦点附近所受的力指向光束焦点。前面讨论了飞秒激光双光子过程的自由基浓度变化过程,但在实际的飞秒激光双光子加工过程中,根据光镊理论,飞秒激光对于活性自由基存在一个指向焦点中心的力,这样会导致实际的加工线宽比之前的理论值要小。因此,飞秒激光双光子加工过程主要涉及两部分: 一是自由基运动过程中的向外扩散,扩散过程产生的位移为S1=FB/(6πμa)t0; 二是活性自由基的集聚效应,产生的位移S2=F/(6πμa)τ[10]。式中,FB为样本溶液中使自由基自由扩散的布朗力,μ为溶液的粘滞系数,a为自由基颗粒的半径,t0为激光脉冲间隔, F为飞秒激光光镊力[11]
F=nPc[Rsin2θ−T2sin(2θ−2φ)+Rsin2θ1+R2+2Rcos2φ] (8) 式中: R, T分别对应入射光束在微粒表面的反射率和折射率; n为介质折射率; θ, φ分别是入射角和反射角。由式(8)可以看出, 光镊力的大小和自由基的相对位置以及激光的功率有关。飞秒激光光镊作用于固化之前的活性自由基,使得活性自由基向焦点中心汇聚,而使得最终加工线宽较之初始的线宽有所减小,但是在自由基固化之后,光镊作用便不会使线宽继续减小,因此真正会影响线宽分布的光镊效应仅发生在形成固化自由基之前。那么最终加工线宽
D=d+√2ln2πρthϕ(σ2τ)ρ0(hcλI0)2(S1−S2) (9) 式中: d为未考虑光镊效应时的线宽,第二项为光镊效应对于线宽的影响作用。光镊在固化之前作用于自由基才会引发自由基的位移,√2ln2πρthϕ(σ2τ)ρ0(hcλI0)2为引发自由基固化所需的脉冲数。
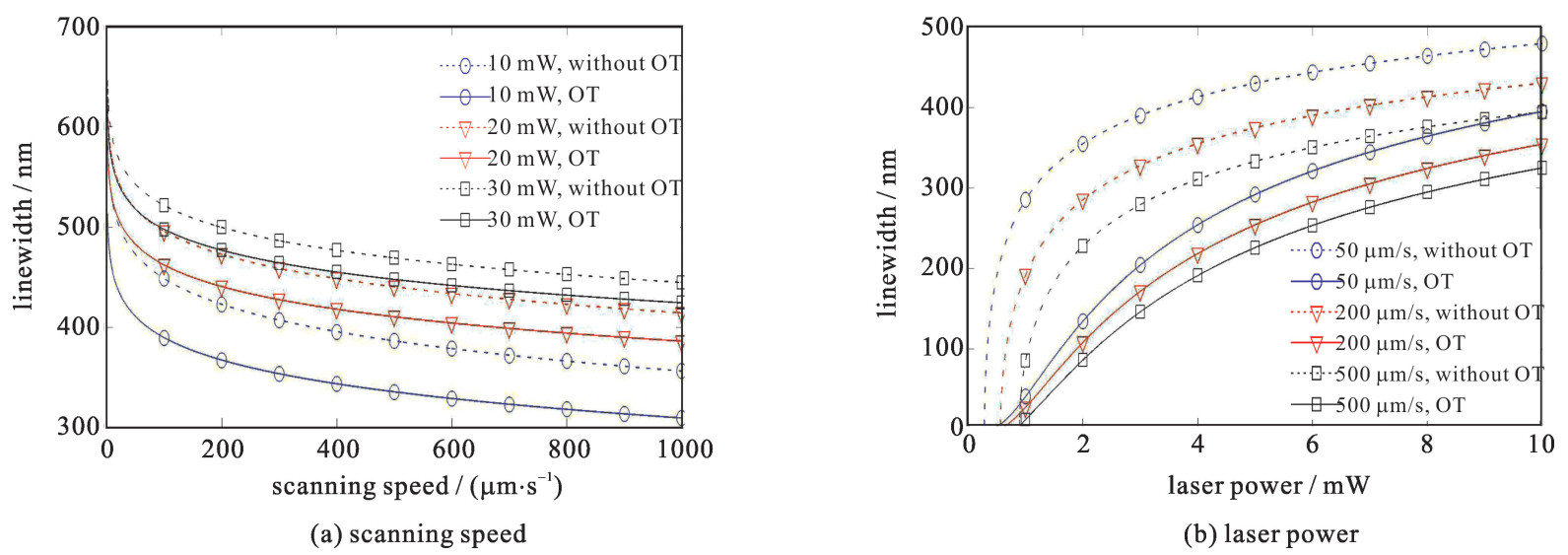
图 3(a)为加工线宽随扫描速度的变化关系,虚线为未考虑光镊(OT)的情况,实线为考虑了光镊之后的加工线宽。此时的激光功率分别控制在10, 20和30 mW,扫描速度由1 μm/s变化至1000 μm/s,可以看出随着扫描速度的变大,加工的线宽不断变小,光镊作用会使加工线宽变小。图 3(b)为加工线宽随激光功率的变化关系,此时控制激光扫描的速度为50, 200和500 μm/s,激光功率由0.1 mW变化至10 mW,虚线为未考虑光镊的情况,实线为考虑了光镊之后的加工线宽,当激光功率小到一定值时,对应的曝光时间内不能产生固化线宽,扫描速度越大,所需对应的聚合临界功率值越大。
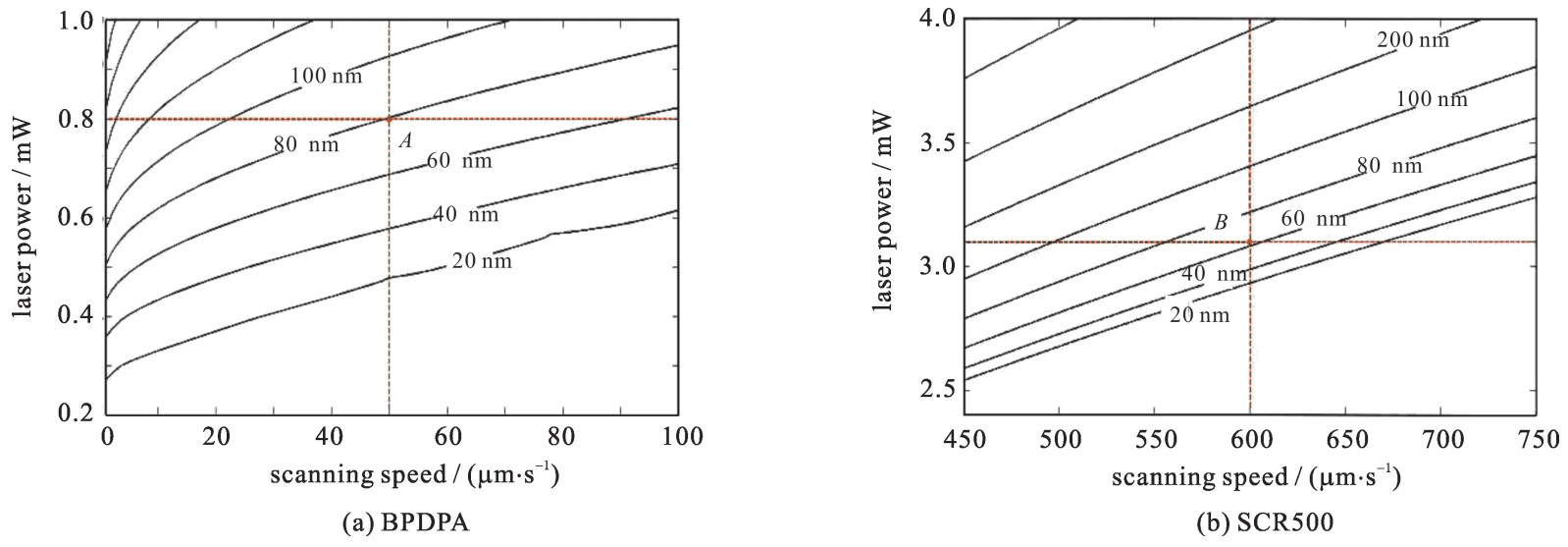
选取波长λ=780 nm,脉宽τ=80 fs,重复频率fq=80 MHz, NA=1.42的钛宝石飞秒激光器。与文献[5]一样,选取吸收截面为σ2=2.94×10-48 cm4·s的材料BPDPA, 根据式(9),得到线宽随扫描速度与激光功率变化的等值线,结果如图 4(a)所示, 其中A点的扫描速度50 μm/s、激光功率为0.8 mW, 由式(9)计算得出线宽为78 nm, 文献[5]中实验加工线宽为80 nm, 两者误差率在2%。选取与图 4(a)相同参数, 在不考虑光镊的情况下,根据式(7)计算得到的线宽为225 nm, 与实验结果误差较大。此外,与图 4(a)相同激光参数,选取加工双光子吸收截面为σ2=20×10-50 cm4·s的材料SCR500, 与文献[1]中参数一致,根据式(9),得到线宽随扫描速度与激光功率变化的等值线,结果如图 4(b)所示, 其中B点选取的扫描速度为600 μm/s、激光功率为3.1 mW, 由式(9) 计算得出线宽为62 nm, 文献[1]中实验加工线宽为68 nm, 两者误差率为8%。如果选取相同的参数,在不考虑光镊的情况下,根据公式(7)计算得到的线宽为194 nm, 与实验结果误差较大。因此,图 4(a)和(b)分别验证了文献[5]和[1]对于飞秒激光双光子加工线宽的实验研究结果。同时,在计算飞秒激光双光子加工线宽时,必须考虑光镊效应的影响。
3. 结论
本文分析双光子聚合过程中自由基的形成以及飞秒激光多次脉冲作用导致自由基分布的变化,接着考虑光镊作用对于活性自由基分布的影响以及线宽的变化。研究发现,该线宽理论很好地解释了自由基分布受扫描速度及激光功率影响而变化的规律,并且光镊效应在计算飞秒激光双光子加工线宽时起着必不可少的作用。当激光功率不变时,扫描速度越大,线宽越小,当扫描速度一定时,激光功率越小,线宽越小。每一个激光功率都对应有一个临界的扫描速度值,同样每一个扫描速度也都对应有一个临界的激光功率, 因此并不能获得任意小的加工线宽。当选取扫描速度50 μm/s、激光功率为0.8 mW的飞秒激光加工BPDPA材料时, 计算得到线宽为78 nm, 与文献[1]中的实验结果80 nm比较, 误差率为2%。理论结果能很好地符合文献[1]与[5]实验数据,进一步说明了该理论的正确性,为飞秒激光双光子加工的研究提供了新的思路,为光镊理论的应用研究提供了一定的理论依据。
-
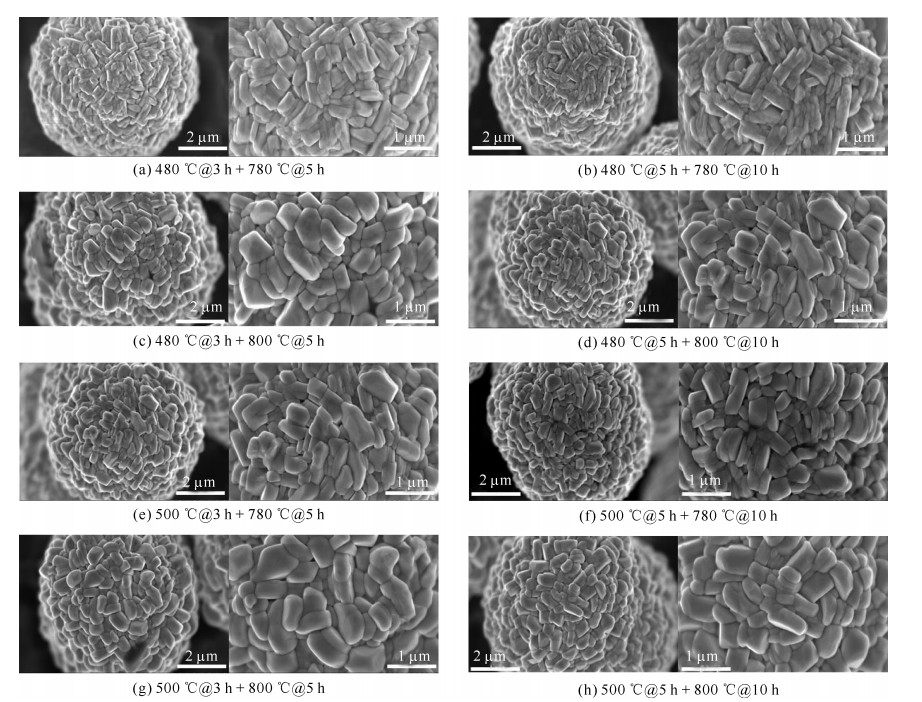
表 1 正极材料LiNi0.8Co0.15Mn0.05O2的烧结工艺
Table 1. Sintering process of cathode material LiNi0.8Co0.15Mn0.05O2
No presintering temperature/℃ presintering time/h calcination temperature/℃ calcination time/h remarks 1 20→480(120 min) 3 480→780 (60 min) 5 cooling 2 20→480(120 min) 5 480→780(60 min) 10 cooling 3 20→480(120 min) 3 480→800(64 min) 5 cooling 4 20→480(120 min) 5 480→800(64 min) 10 cooling 5 20→500(124 min) 3 500→780(56 min) 5 cooling 6 20→500(124 min) 5 500→780(56 min) 10 cooling 7 20→500(124 min) 3 500→800(60 min) 5 cooling 8 20→500(124 min) 5 500→800(60 min) 10 cooling 表 2 不同烧结工艺下正极材料的NCM晶格参数
Table 2. NCM lattice parameters of cathode material under different sintering processes
sample a/nm c/nm c/a I(003) I(104) R=I(003)/I(104) 480 ℃@3 h+780 ℃@5 h 0.250 74 1.409 47 5.621 2 100 54.2 1.845 0 480 ℃@3 h+800 ℃@5 h 0.250 48 1.410 10 5.629 6 100 51.0 1.960 8 500℃@3 h+780 ℃@5 h 0.286 45 1.410 94 4.925 6 100 57.4 1.742 2 500℃@3 h+800 ℃@5 h 0.286 38 1.407 10 4.913 4 100 58.9 1.697 8 480 ℃@5 h+780 ℃@10 h 0.250 39 1.410 24 5.632 2 100 54.9 1.821 5 480 ℃@5 h+800 ℃@10 h 0.250 56 1.409 18 5.624 1 100 63.1 1.584 8 500℃@5 h+780 ℃@10 h 0.286 20 1.407 21 4.916 9 100 47.6 2.100 8 500℃@5 h+800 ℃@10 h 0.286 37 1.411 11 4.927 6 100 57.5 1.739 1 表 3 不同烧结工艺下正极材料EIS拟合结果
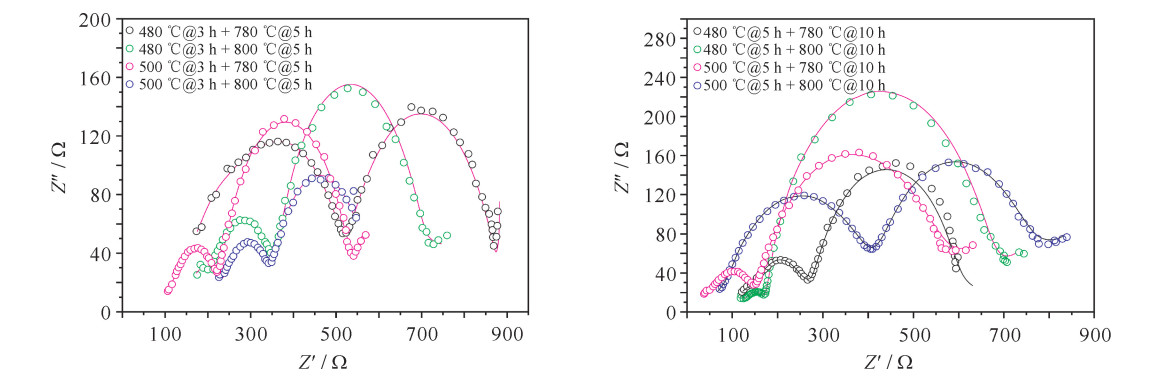
Table 3. EIS fitting results of cathode material under different sintering processes
sample electrolyte resistivity/Ω RSEI/Ω resistency/Ω Rct/Ω 480 ℃@3 h+780 ℃@5 h 135.40 171.70 211.50 366.20 480 ℃@3 h+800 ℃@5 h 153.50 310.60 146.60 44.84 500℃@3 h+780 ℃@5 h 90.93 43.19 100.30 293.60 500℃@3 h+800 ℃@5 h 0.001 253 237.40 218.50 111.10 480 ℃@5 h+780 ℃@10 h 3.933 271.00 86.11 20.65 480 ℃@5 h+800 ℃@10 h 0.544 1 31.76 134.50 460.50 500℃@5 h+780 ℃@10 h 17.00 36.86 360.60 80.89 500℃@5 h+800 ℃@10 h 27.21 312.7 47.51 276.70 表 4 各材料CV曲线对应的主要氧化还原峰电位及电位差
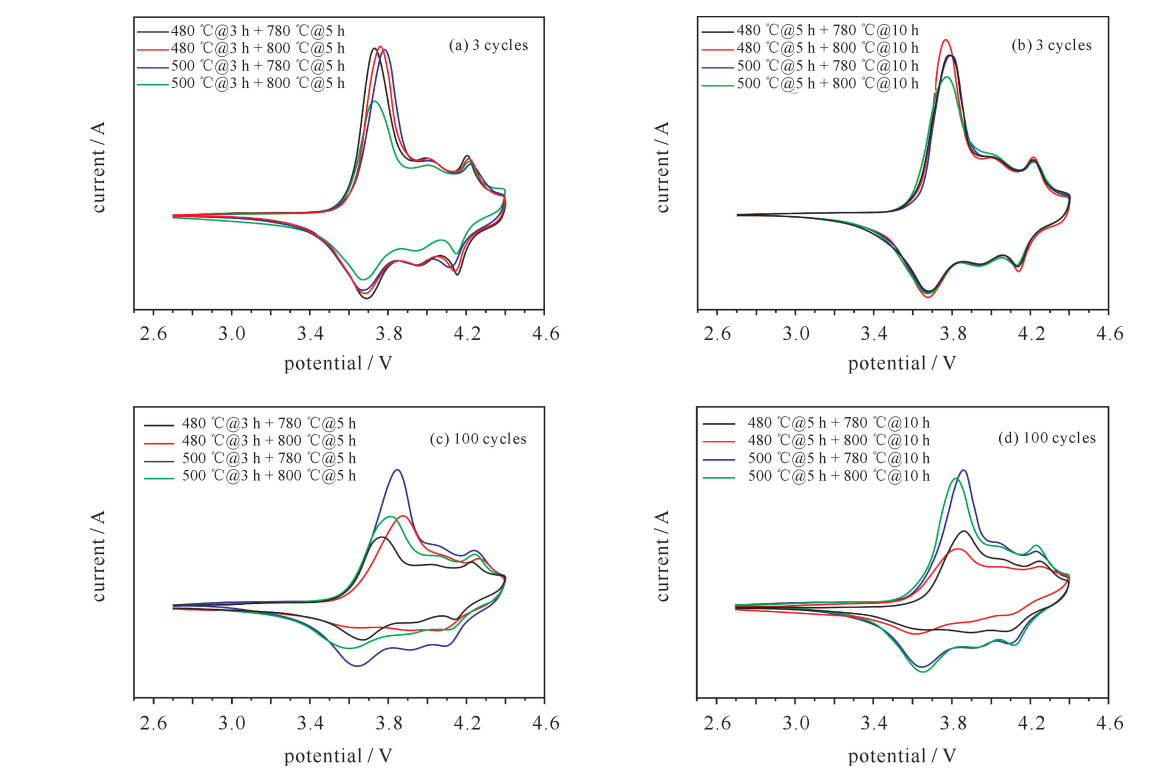
Table 4. Main REDOX peak potential and PD corresponding to CV curve of each material
sample oxidation peak potential/V reduction peak potential/V potential(oxidation peak-reduction peak)/V 3 cycles 100 cycles 3 cycles 100 cycles 3 cycles 100 cycles 480 ℃@3 h+780 ℃@5 h 3.733 3.767 3.699 3.669 0.034 0.098 480 ℃@3 h+800 ℃@5 h 3.760 3.872 3.682 3.628 0.078 0.244 500℃@3 h+780 ℃@5 h 3.784 3.846 3.670 3.639 0.114 0.207 500℃@3 h+800 ℃@5 h 3.732 3.817 3.667 3.592 0.065 0.225 480 ℃@5 h+780 ℃@10 h 3.776 3.859 3.681 3.653 0.095 0.206 480 ℃@5 h+800 ℃@10 h 3.769 3.828 3.680 3.626 0.089 0.202 500℃@5 h+780 ℃@10 h 3.794 3.859 3.679 3.644 0.115 0.215 500℃@5 h+800 ℃@10 h 3.780 3.823 3.680 3.652 0.100 0.171 表 5 三元正极材料电化学性能对比表
Table 5. Properties comparison of ternary layered oxide cathode materials
ternary anode material initial charge-discharge specific capacity at 0.1C magnification/(mA·h·g-1) capacity retention ratio after 100 cycles at 1C/% ref. LiNi0.6Co0.2Mn0.2O2 173.0 89.7 [23] LiNi0.5Co0.2Mn0.3O2 167.4 89.7 [24] LiNi1/3Co1/3Mn1/3O2 152.3 85.8 [25] LiNi0.4Co0.2Mn0.4O2 180.7 90.3 [26] LiNi1-x-yCoxAlyO2 172.7 92.1 [27] LiNi0.8Co0.15Mn0.05O2 200.0 94.0 -
[1] 闫金定. 锂离子电池发展现状及其前景分析[J]. 航空学报, 2014, 35(10): 2767-2775. https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htmYan Jinding. Current status and development analysis of lithium-ion batteries. Acta Aeronautica et Astronautica Sinica, 2014, 35(10): 2767-2775 https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htm [2] Li Yangxing, Wan Chunrong, Wu Yuping, et al. Synthesis and characterization of ultrafine LiCoO2 powders by a spray-drying method[J]. Journal of Power Sources, 2000, 85(2): 294-298. doi: 10.1016/S0378-7753(99)00159-7 [3] Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451: 652-657. doi: 10.1038/451652a [4] 庞春会, 吴川, 吴锋, 等. 锂离子电池纳米正极材料合成方法研究进展[J]. 硅酸盐学报, 2012, 40(2): 247-255. doi: 10.14062/j.issn.0454-5648.2012.02.012Pang Chunhui, Wu Chuan, Wu Feng, et al. Development on synthesis methods for nano-materials of cathode for lithium ion battery. Journal of the Chinese Ceramic Society, 2012, 40(2): 247-255 doi: 10.14062/j.issn.0454-5648.2012.02.012 [5] Patil A, Patil V, Shin D W, et al. Issue and challenges facing rechargeable thin film lithium batteries[J]. Mater Res Bull, 2008, 43: 1913-1942. doi: 10.1016/j.materresbull.2007.08.031 [6] Shukla A K, Kumar T P. Materials for next-generation lithium batteries[J]. Curr Sci, 2008, 94: 314-331. [7] Hassoun J, Kim J H, Lee D J. A contribution to the progress of high energy batteries: A metal-free, lithium-ion, silicon-sulfur battery[J]. Journal of Power Sources, 2012, 202: 308-313. doi: 10.1016/j.jpowsour.2011.11.060 [8] 邹邦坤, 丁楚雄, 陈春华. 锂离子电池三元正极材料的研究进展[J]. 中国科学: 化学, 2014, 44(7): 1104-1115. https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htmZou Bangkun, Ding Chuxiong, Chen Chunhua. Research progress in ternary cathode materials Li(Ni, Co, Mn)O2 for lithium ion batteries. Scientia Sinica Chimica, 2014, 44(7): 1104-1115 https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htm [9] 黄可龙, 王兆翔, 刘素琴. 锂离子电池原理与关键技术[M]. 北京: 化学工业出版社, 2008: 1-10.Huang Kelong, Wang Zhaoxiang, Liu Suqin. Principle and key technology of lithium ion battery. Beijing: Chemical Industry Press, 2008: 1-10 [10] 马璨, 吕迎春, 李泓. 锂离子电池基础科学问题(Ⅶ)—正极材料[J]. 储能科学与技术, 2014, 3(1): 53-65. doi: 10.3969/j.issn.2095-4239.2014.01.008Ma Can, Lü Yingchun, Li Hong. Fundamental scientific aspects of lithium batteries (Ⅶ)—positive electrode materials. Energy Storage Science and Technology, 2014, 3(1): 53-65 doi: 10.3969/j.issn.2095-4239.2014.01.008 [11] 章福平, 纪勇, 李安东, 等. 锂离子电池正极材料研究的新动向和挑战[J]. 化学通报, 2011, 74(10): 891. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htmZhang Fuping, Ji Yong, Li Andong, et al. Progresses and challenges in studies on cathodic materials of Li-ion battery. Chemistry, 2011, 74(10): 891 https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htm [12] Wang Zhao. Studies on Li-excess Mn-based Li1. 2Mn0.54Ni0.13Co0.13O2 as cathode materials for lithium batteries. Beijing: Beijing Institute of Technology, 2015: 1-189 [13] 周燕芳, 钟辉. 锂离子电池正极材料的研究进展[J]. 材料开发与应用, 2003, 18(2): 34-38. doi: 10.3969/j.issn.1003-1545.2003.02.012Zhou Yanfang, Zhong Hui. Research progress in positive electrode materials for lithium ion battery. Development and Application of Materials, 2003, 18(2): 34-38 doi: 10.3969/j.issn.1003-1545.2003.02.012 [14] Muto S S, Tatsumi K S, Kojima Y J, et al. Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods[J]. Power Sources, 2012, 205: 449-455. doi: 10.1016/j.jpowsour.2012.01.071 [15] Cui Y L, Bao W J, Yuan Z, et al. Comparison of different soft chemical routes synthesis of submicro-LiMn2O4 and their influence on its electrochemical properties[J]. Solid State Electrochemistry, 2012, 16(4): 1551-1559. doi: 10.1007/s10008-011-1558-6 [16] 周文彩, 李金洪, 姜晓谦. 磷酸铁锂制备工艺及研究进展[J]. 硅酸盐通报, 2010, 29(1): 134-146. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htmZhou Wencai, Li Jinhong, Jiang Xiaoqian. Preparation and research progress of LiFePO4 cathode material. Bulletin of the Chinese Ceramic Society, 2010, 29(1): 134-146 https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htm [17] 张新龙, 胡国荣, 彭忠东, 等. 锂离子电池正极材料LiFePO4的研究进展[J]. 电池, 2003, 33(4): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htmZhang Xinlong, Hu Guo-rong, Peng Zhongdong, et al. Development of LiFePO4 as cathode material of Li-ion battery. Battery Bimonthly, 2003, 33(4): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htm [18] 沙鸥, 赵敏寿, 翟静, 等. 锂离子电池新型正极材料LiFePO4的研究进展[J]. 稀有金属材料与工程, 2009, 38(11): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htmSha Ou, Zhao Minshou, Zhai Jing, et al. Research progress on LiFePO4—A new type of cathode materials for lithium-ion battery. Rare Metal Materials and Engineering, 2009, 38(11): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htm [19] 翟静, 赵敏寿, 沙鸥, 等. 锂离子电池正极材料Li3V2(PO4)3的研究进展[J]. 稀有金属材料与工程, 2010, 39(7): 1311-1316. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htmZhai Jing, Zhao Minshou, Sha Ou, et al. Research progress in cathode material Li3V2(PO4)3 for Li-ion batteries. Rare Metal Materials and Engineering, 2010, 39(7): 1311-1316 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htm [20] Li Y J, Hong L, Wu F. Preparation of Li3V2(PO4)3 cathode material for power Li-ion batteries[J]. Progress in Chemistry, 2012, 24(1): 47-53. [21] 李龙. 锂离子电池镍钴锰三元正极材料的合成与改性研究[D]. 北京: 清华大学, 2012: 1-54.Li Long. Synthesis and modification of LiNixCoyMnzO2 cathode material for lithium battery. Beijing: Tsinghua University, 2012: 1-54 [22] Ruan Zewen. Synthesis and modification of LiNi1-x-yCoxAlyO2 nickel rich cathode materials. Harbin: Harbin Institute of Technology, 2016: 1-81 [23] 艾灵, 毛丽萍, 李世友, 等. 三元正极材料LiNi0.6Co0.2Mn0.2O2制备及改性方法进展[J]. 化工新型材料, 2018, 46(5): 11-15. https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htmAi Ling, Mao Liping, Li Shiyou, et al. Progress on the preparation and modification of LiNi0.6Co0.2Mn0.2O2 ternary anode material. New Chemical Materials, 2018, 46(5): 11-15 https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htm [24] 李尚津. 锂离子电池三元正极材料LiNi0.5Co0.2Mn0.3O2的制备与改性[D]. 南宁: 广西大学: 2017: 1-47.Li Shangjin. Preparation and modification of LiNi0.5Co0.2Mn0.3O2 of the lithium ion battery. Nanning: Guangxi University, 2017: 1-47 [25] 路乃群, 王存国, 王静强, 等. 锂离子电池三元正极材料LiNi1/3Co1/3Mn1/3O2的制备方法及其改性研究进展[J]. 化工科技, 2018, 26(6): 58-63. https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htmLu Naiqun, Wang Cunguo, Wang Jingqiang, et al. Development of the ternary cathode material LiNi1/3Co1/3Mn1/3O2 and its modification for lithium ion batteries. Science & Technology in Chemical Industry, 2018, 26(6): 58-63 https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htm [26] Wolff-Goodrich S, Lin F, Markus I M, et al. Tailoring the surface properties of LiNi0.4Mn0.4Co0.2O2 by titanium substitution for improved high voltage cycling performance[J]. Physical Chemistry Chemical Physics, 2015, 17(34): 21778-21781. [27] 王义飞, 武行兵, 王双双, 等. 三元正极材料LiNi1- x- yCoxAlyO的研究进展[J]. 电池, 2017, 47(2): 112-115. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htmWang Yifei, Wu Xingbing, Wang Shuangshuang, et al. Research progress in ternary cathode materials LiNi1-x-yCoxAlyO2. Battery Bimonthly, 2017, 47(2): 112-115 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htm 期刊类型引用(0)
其他类型引用(3)
-






 下载:
下载:



 下载:
下载: