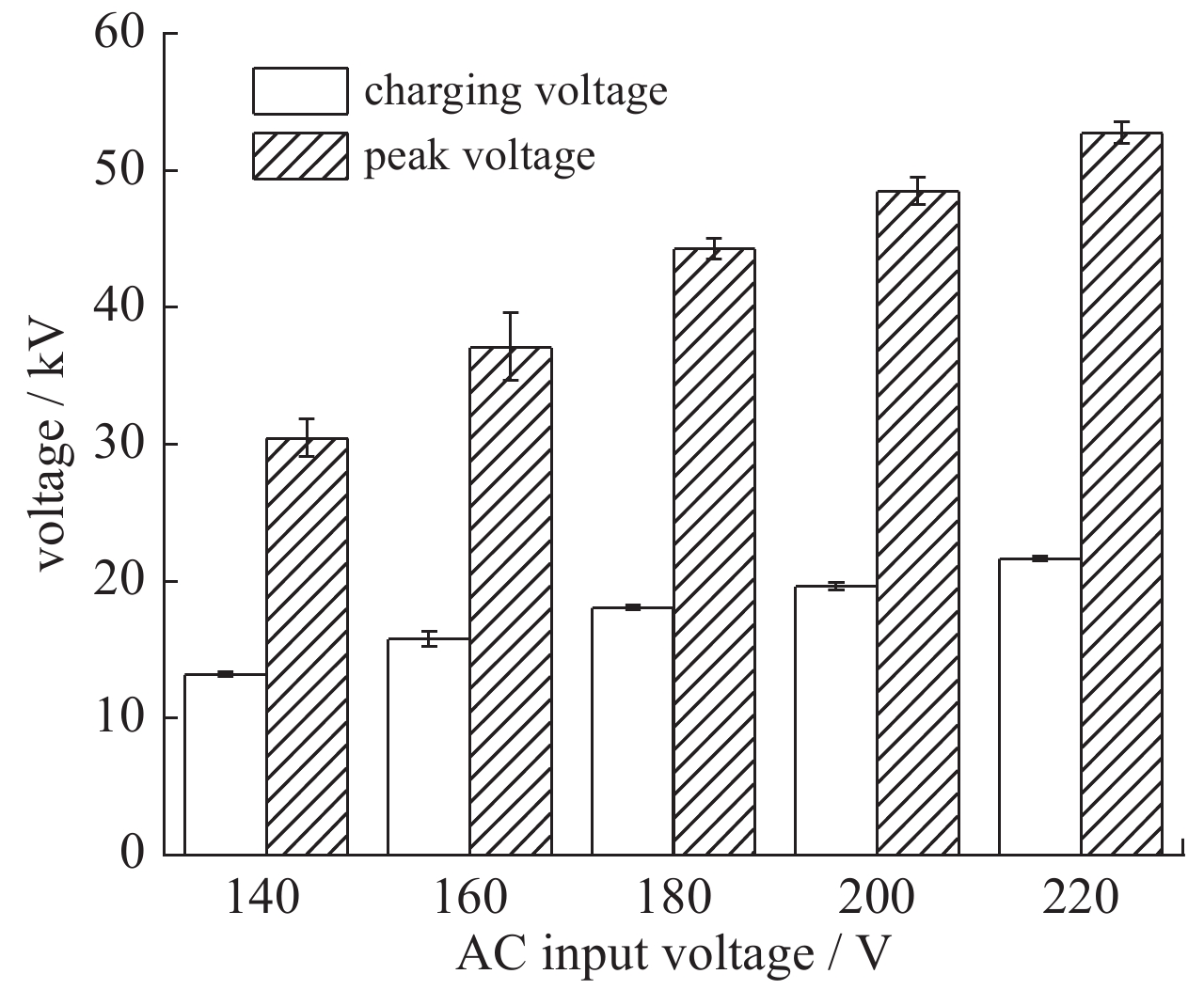
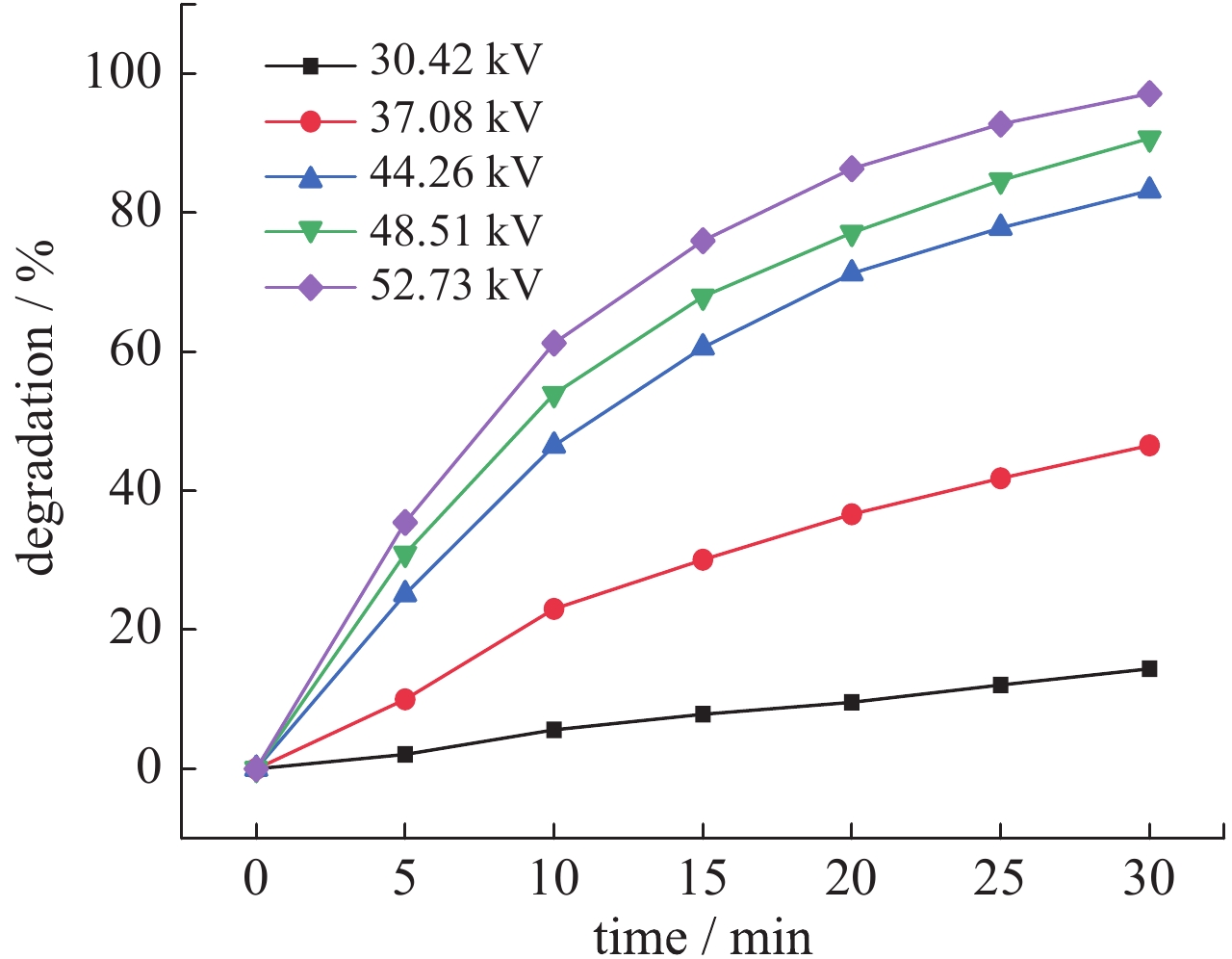
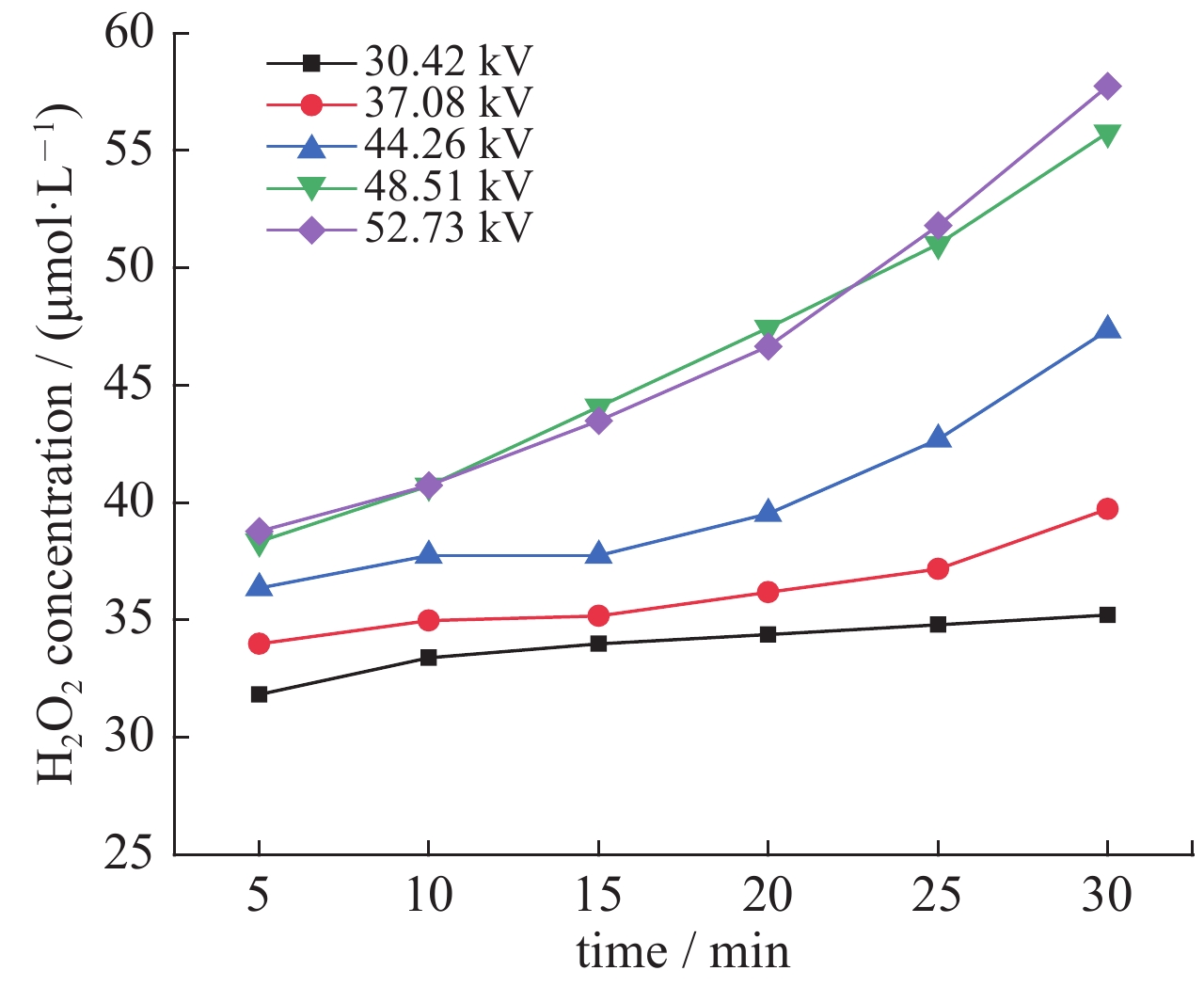
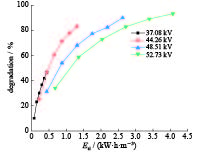
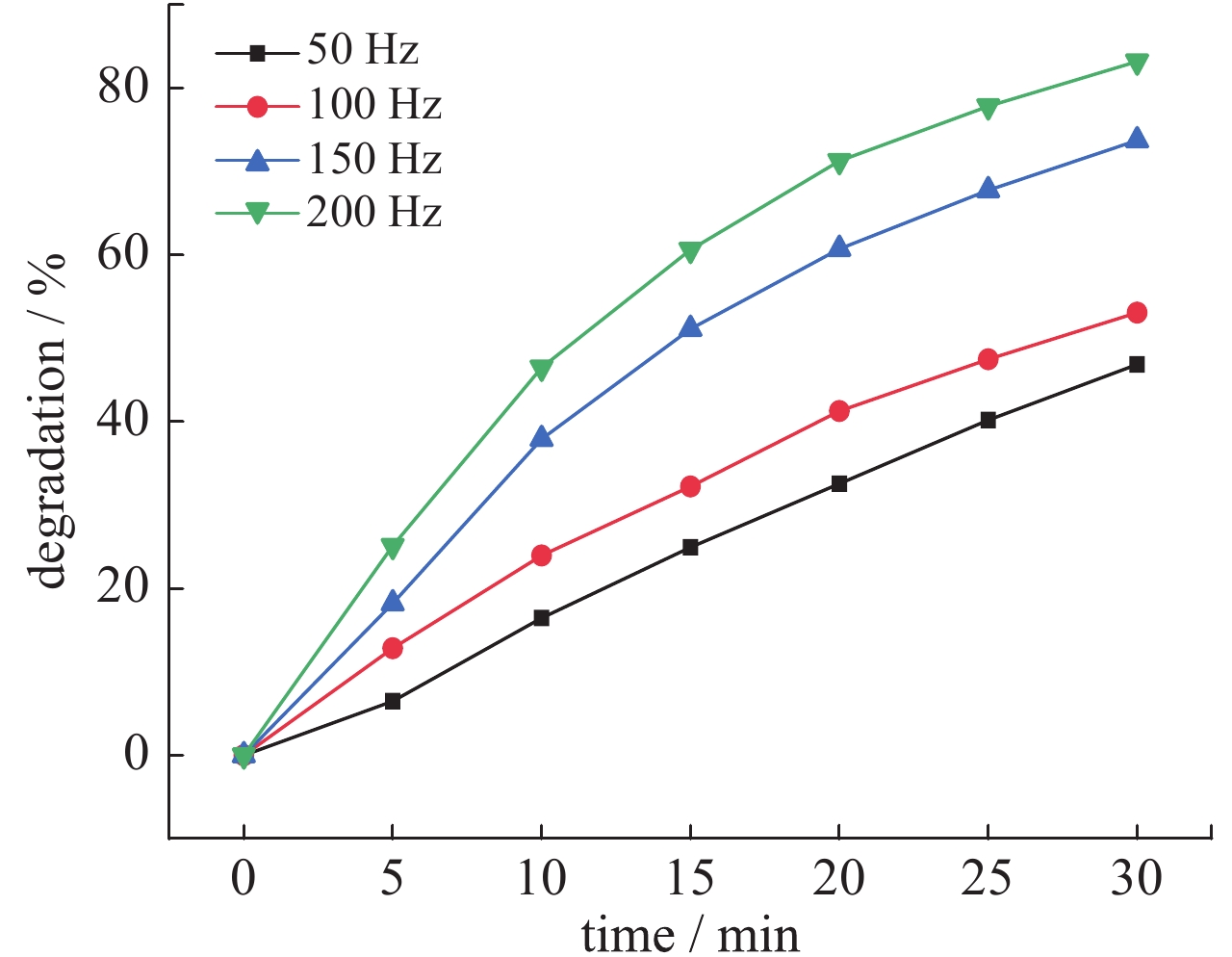
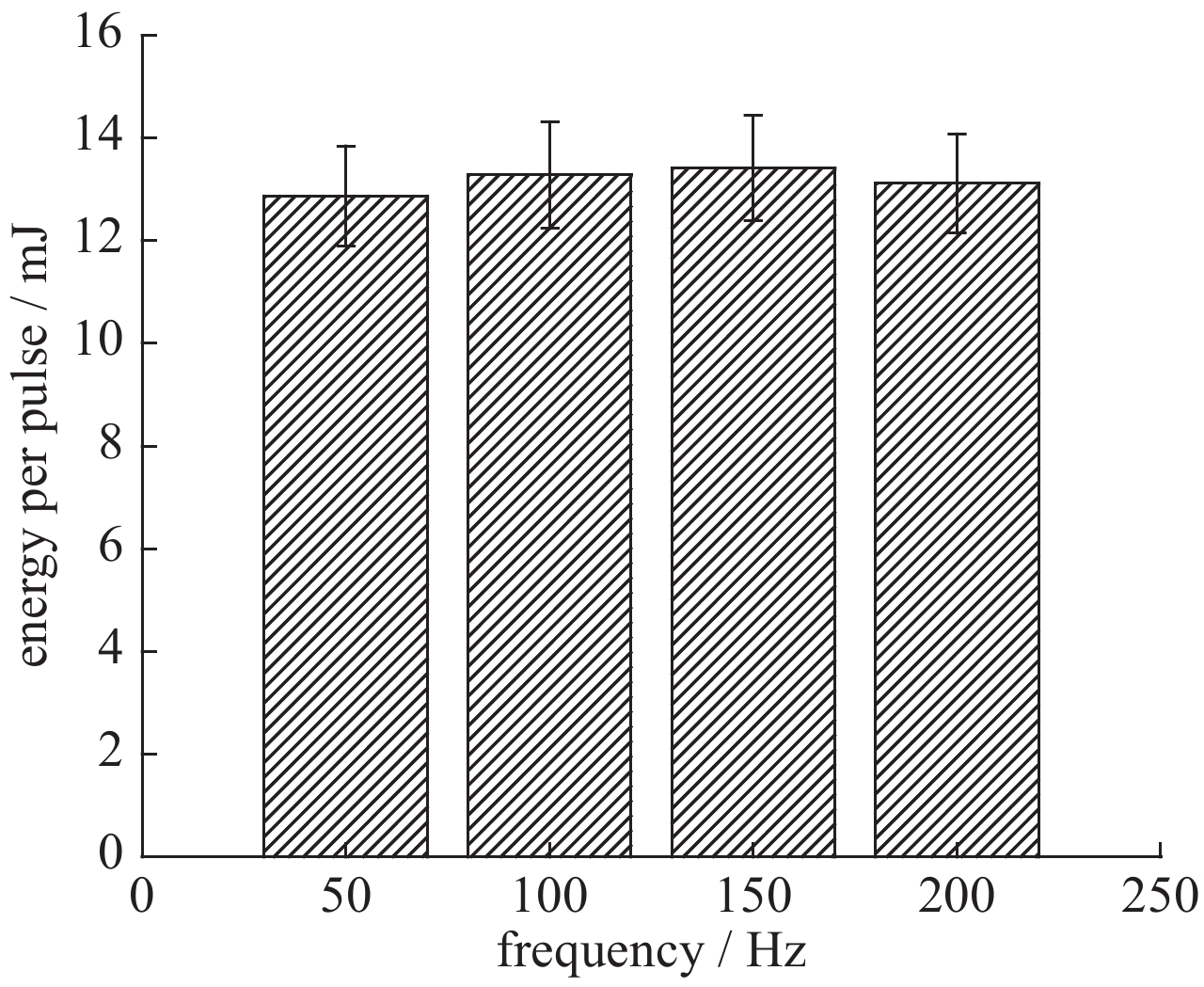
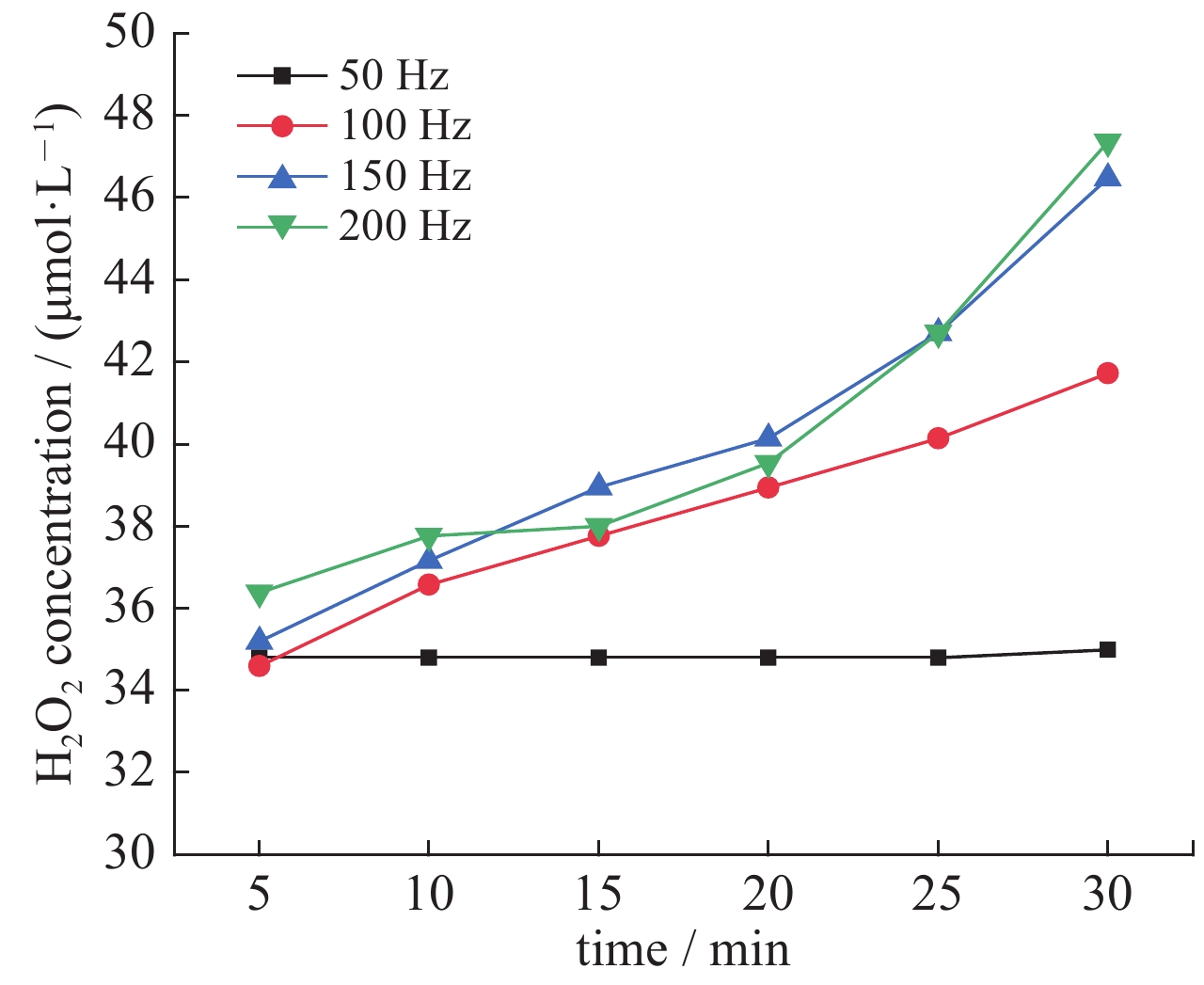
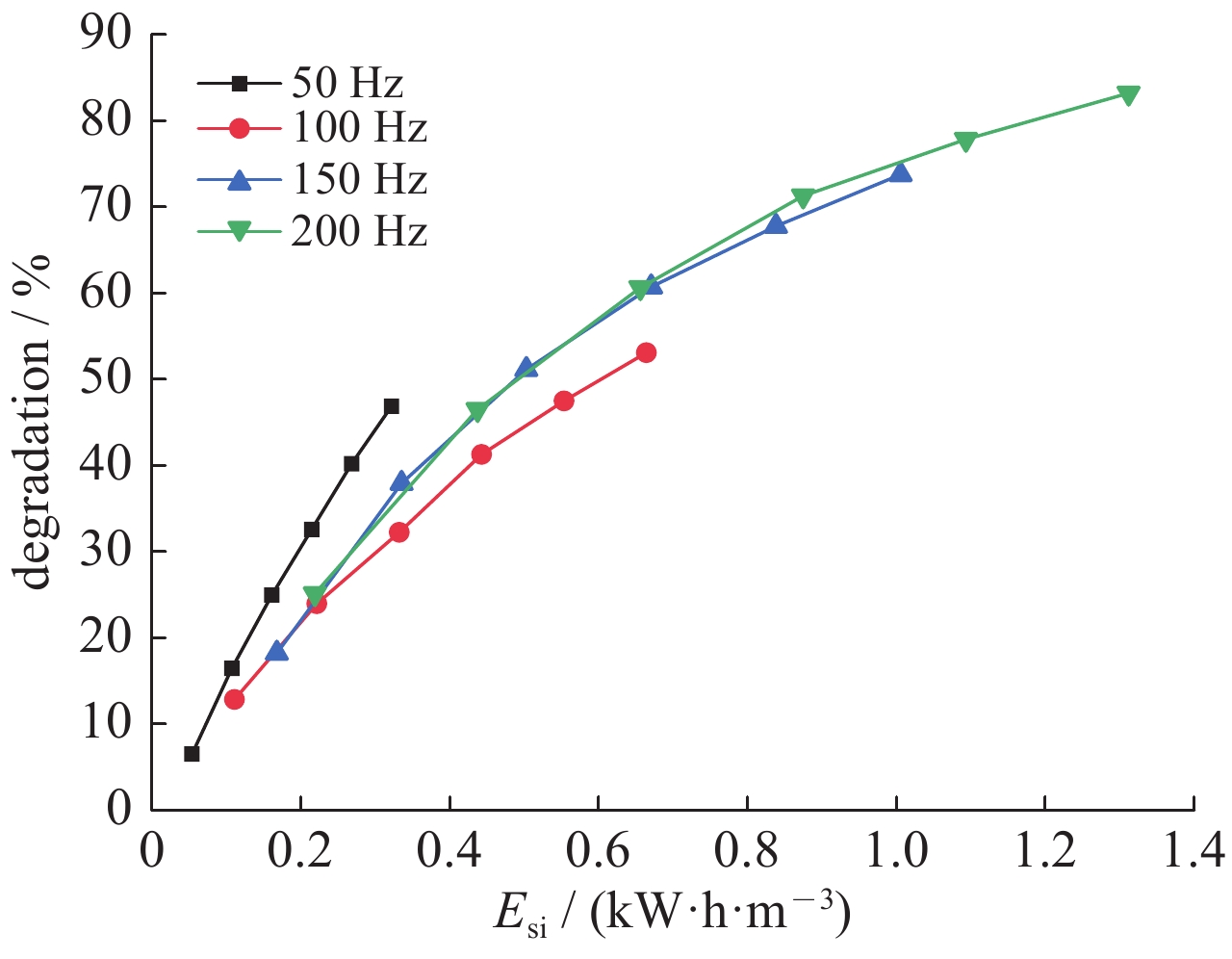
| [1] |
李家珍. 染料, 染色工业废水处理[M]. 北京: 化学工业出版社, 1997.Li Jiazhen. Dyes, dyeing industrial wastewater treatment. Beijing: Chemical Industry Press, 1997
|
| [2] |
Yang Shiying, Wang Ping, Yang Xin, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide[J]. Journal of Hazardous Materials, 2010, 179(1/3): 552-558.
|
| [3] |
Wang Q, Gao F, Yuan X, et al. Electrochemical studies on the binding of a carcinogenic anthraquinone dye, Purpurin (C.I.58 205) with DNA[J]. Dyes and Pigments, 2010, 84(3): 213-217. doi: 10.1016/j.dyepig.2009.09.004
|
| [4] |
Chequer F M D, Angeli J P F, Ferraz E R A, et al. The azo dyes Disperse Red 1 and Disperse Orange 1 increase the micronuclei frequencies in human lymphocytes and in HepG2 cells[J]. Mutation Research - Genetic Toxicology and Environmental Mutagenesis, 2009, 676(1): 83-86.
|
| [5] |
Gao J, Wang X, Hu Z, et al. Plasma degradation of dyes in water with contact glow discharge electrolysis[J]. Water Research, 2003, 37(2): 267-272. doi: 10.1016/S0043-1354(02)00273-7
|
| [6] |
闫金霞, 雷庆铎, 王燕. 铁屑-烟道灰内电解法处理模拟分散大红GS染料废水[J]. 化工环保, 2010, 30(3):196-198. (Yan Jinxia, Lei Qingduo, Wang Yan. Treatment of simulated dye wastewater containing disperse Scarlet GS by scrap iron-flue dust internal electrolysis process[J]. Environmental Protection of Chemical Industry, 2010, 30(3): 196-198 doi: 10.3969/j.issn.1006-1878.2010.03.003
|
| [7] |
Francis, Lu ck. Wet air oxidation: past, present and future[J]. Catalysis Today, 1999, 53: 81-91. doi: 10.1016/S0920-5861(99)00112-1
|
| [8] |
Thornton T D, Ladue D E, Savage P E. Phenol oxidation in supercritical water: Formation of dibenzofuran, dibenzo-p-dioxin, and related compounds[J]. Environmental Science and Technology, 1991, 25(8): 1507-1510. doi: 10.1021/es00020a023
|
| [9] |
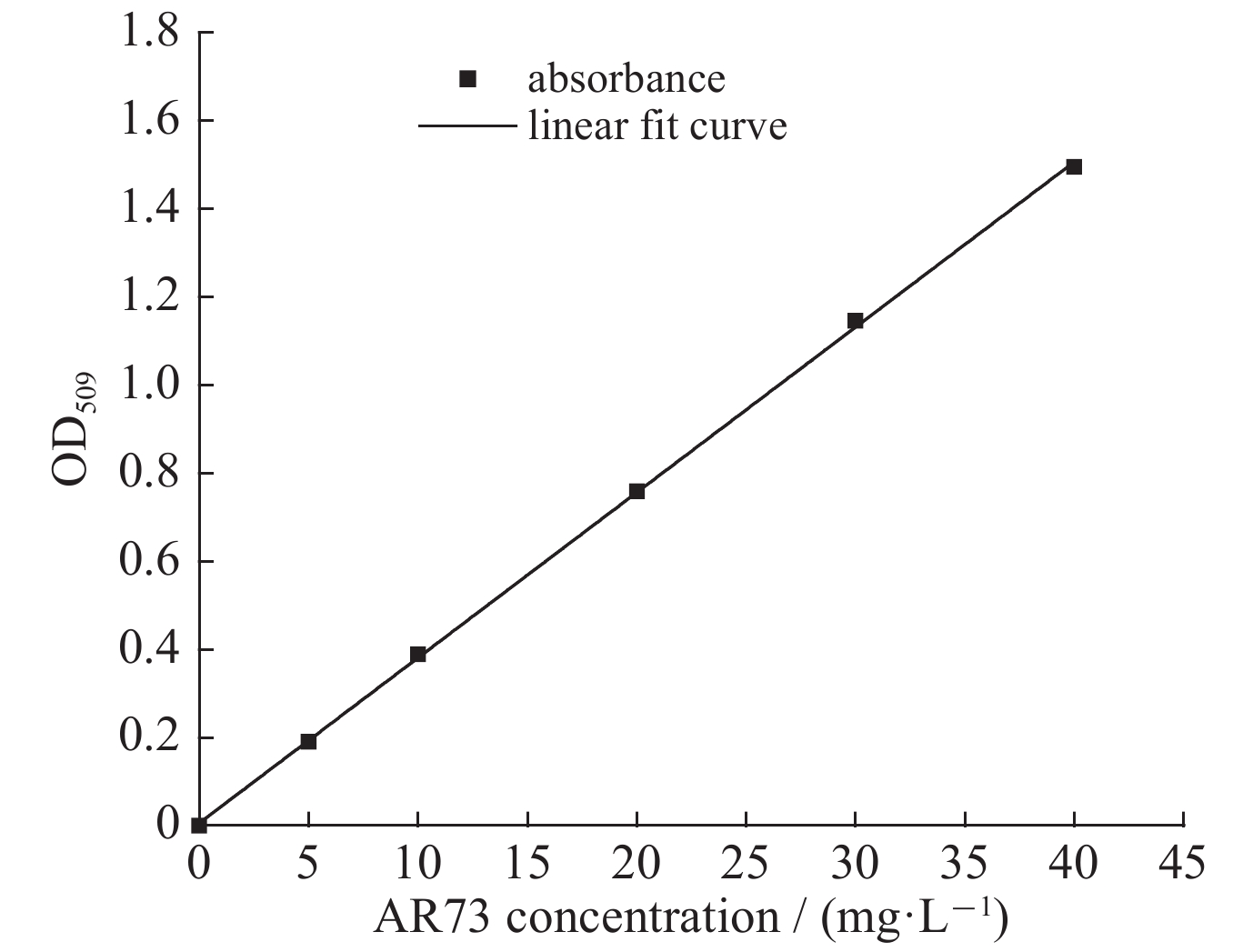
Fu F, Wang Q, Tang B. Effective degradation of C.I. Acid Red 73 by advanced Fenton process[J]. Journal of Hazardous Materials, 2010, 174(1/3): 17-22.
|
| [10] |
Crittenden J C, Liu J, Hand D W, et al. Photocatalytic oxidation of chlorinated hydrocarbons in water[J]. Water Research, 1997, 31(3): 429-438. doi: 10.1016/S0043-1354(96)00267-9
|
| [11] |
张旋, 王启山. 高级氧化技术在废水处理中的应用[J]. 水处理技术, 2009, 35(3):18-22. (Zhang Xuan, Wang Qishan. Application of advanced oxidation technologies in wastewater treatment[J]. Technology of Water Treatment, 2009, 35(3): 18-22
|
| [12] |
王应红. 低温等离子体化学及其应用[J]. 乐山师范学院学报, 2007, 22(5):25-28. (Wang Yinghong. Low temperature plasma chemistry and application[J]. Journal of Leshan Normal University, 2007, 22(5): 25-28 doi: 10.3969/j.issn.1009-8666.2007.05.013
|
| [13] |
刘丹, 张连成, 黄逸凡, 等. 双杆介质阻挡放电降解酸性红73废水[J]. 化工进展, 2018, 37(9):3640-3648. (Liu Dan, Zhang Liancheng, Huang Yifan, et al. Degradation of Brilliant Crocein wastewater by double-rod dielectric barrier discharge[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3640-3648
|
| [14] |
Magureanu M, Bradu C, Piroi D, et al. Pulsed corona discharge for degradation of methylene blue in water[J]. Plasma Chemistry and Plasma Processing, 2013, 33(1): 51-64. doi: 10.1007/s11090-012-9422-8
|
| [15] |
Yan K, Veldhuizen V. Flue gas cleaning by pulse corona streamer[R]. 93-E-272, 1993.
|
| [16] |
Yan K, Liu Z. An industrial streamer corona plasma system for gas cleaning[J]. IEEE Trans Plasma Science, 2006, 34(5): 2426-2433. doi: 10.1109/TPS.2006.881278
|
| [17] |
Yan K, Van Heesch E J M, Pemen A J M, et al. A high-voltage pulse generator for corona plasma generation[J]. IEEE Trans Industry Applications, 2002, 38(3): 866-872. doi: 10.1109/TIA.2002.1003442
|
| [18] |
Venkata S S, Krishnamurthy S. Transient electronics: pulsed circuit technology[J]. IEEE Power and Energy Magazine, 2003, 1(2): 64-64. doi: 10.1109/MPAE.2003.1192028
|
| [19] |
王松松, 杨汉武. 一种四级传输线脉冲变压器的初步研究[J]. 强激光与粒子束, 2009, 21(4):633-636. (Wang Songsong, Yang Hanwu. Preliminary study of a 4-stage transmission line transformer[J]. High Power Laser and Particle Beams, 2009, 21(4): 633-636
|
| [20] |
Hoeben W F L M, Van Veldhuizen E M, Rutgers W R, et al. The degradation of aqueous phenol solutions by pulsed positive corona discharges[J]. Plasma Sources Science and Technology, 2002, 9(3): 361-369.
|



 下载:
下载: