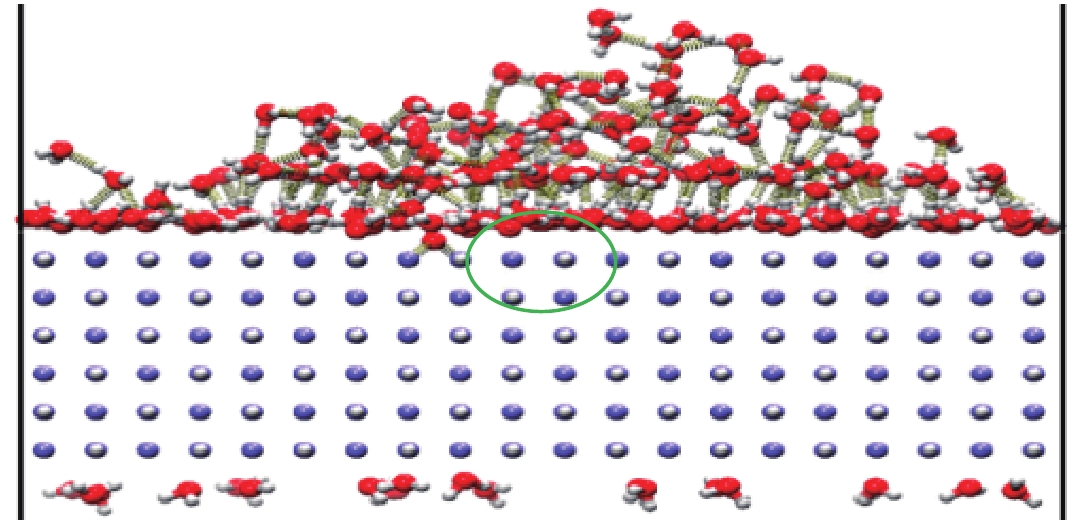
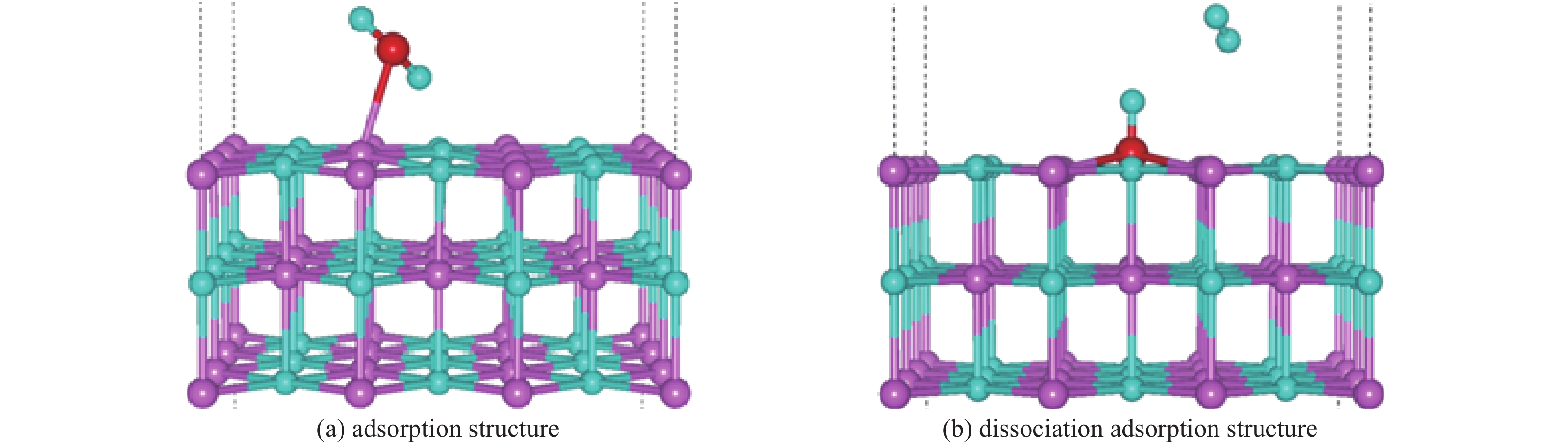
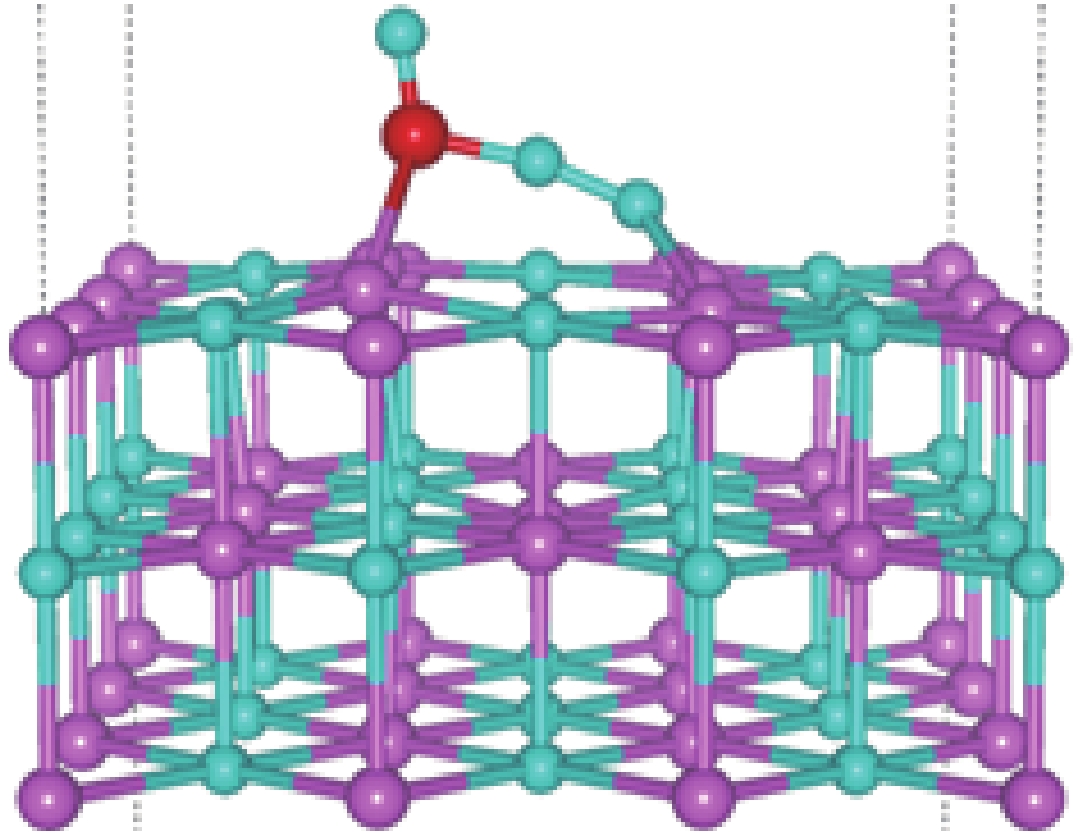
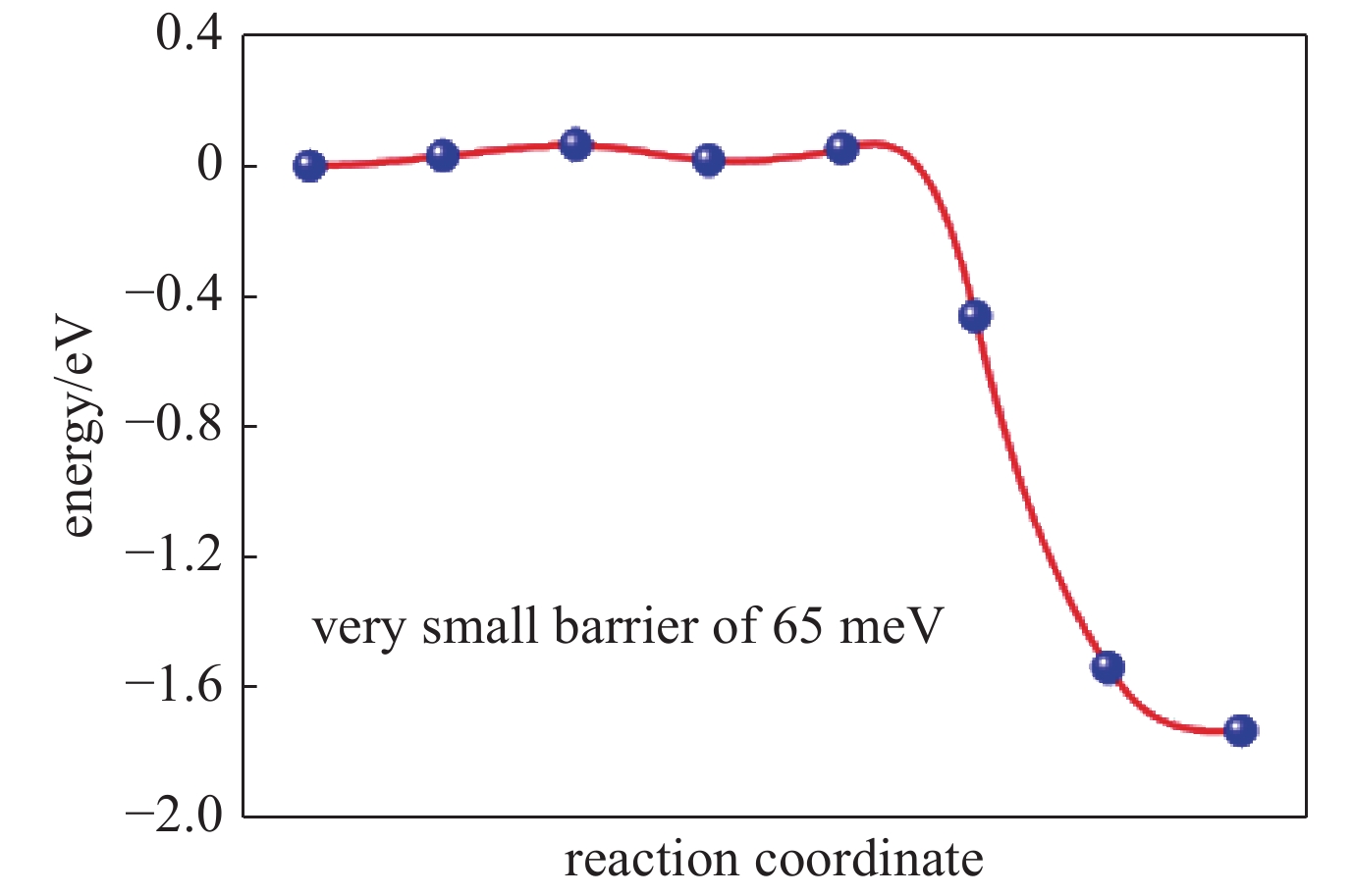
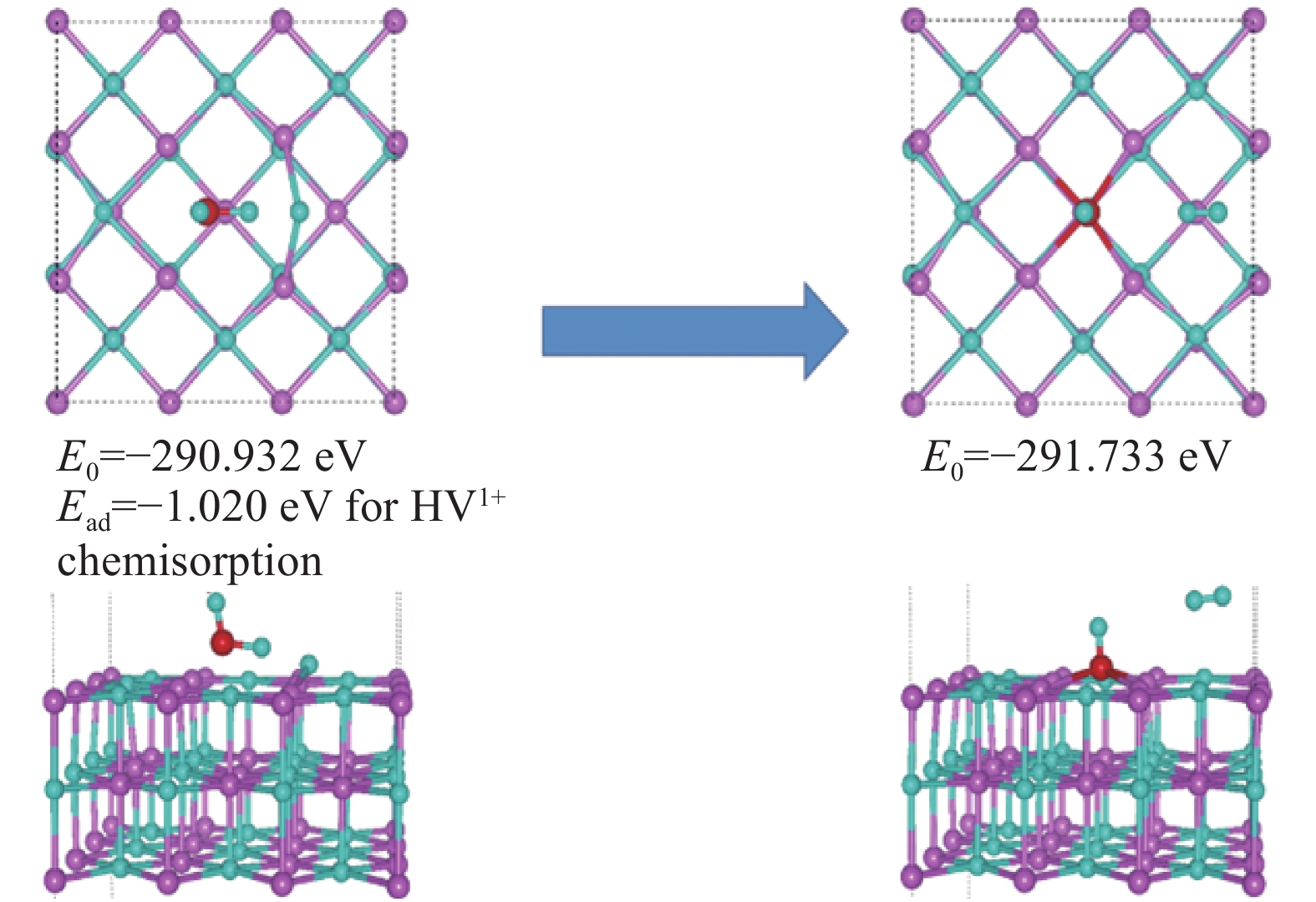
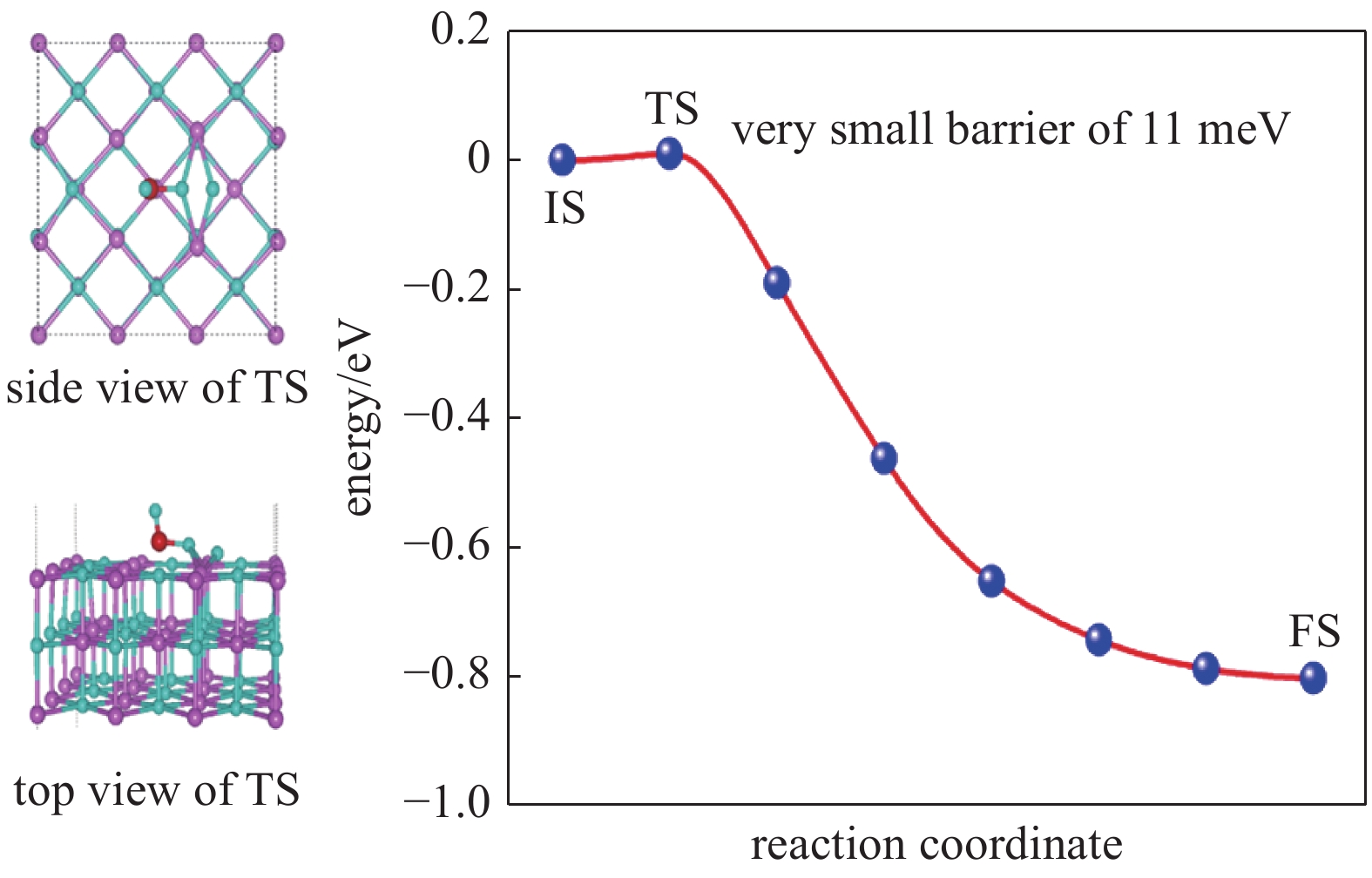
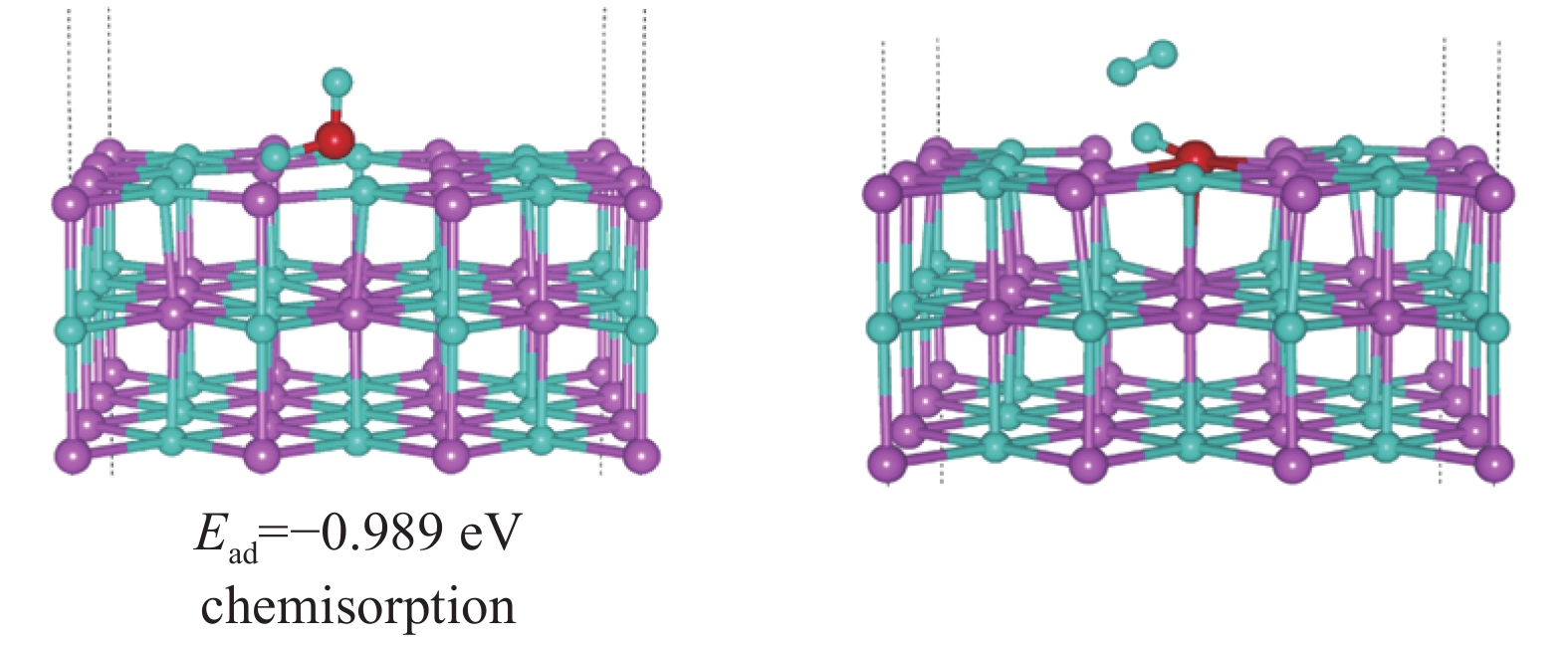
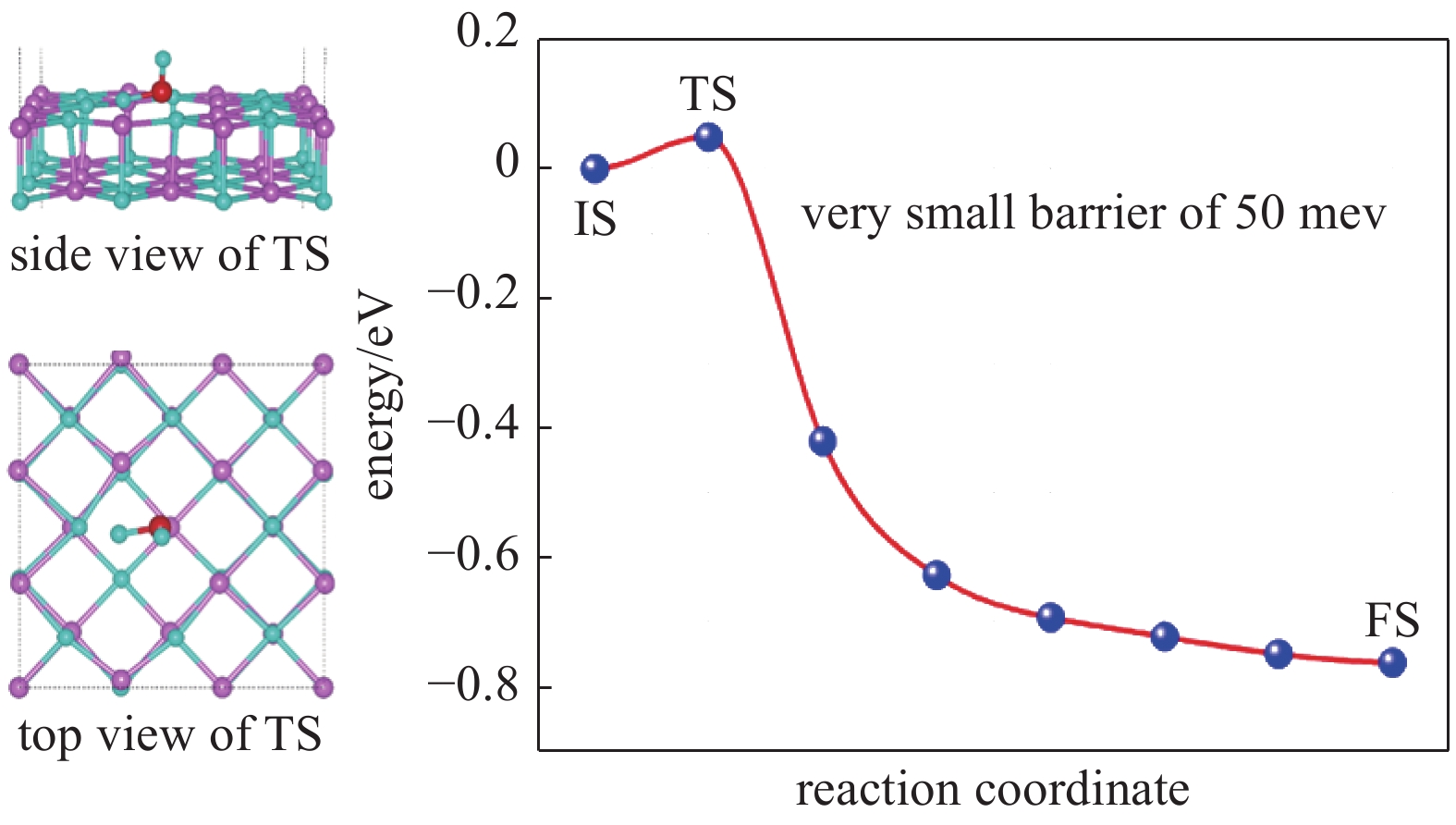
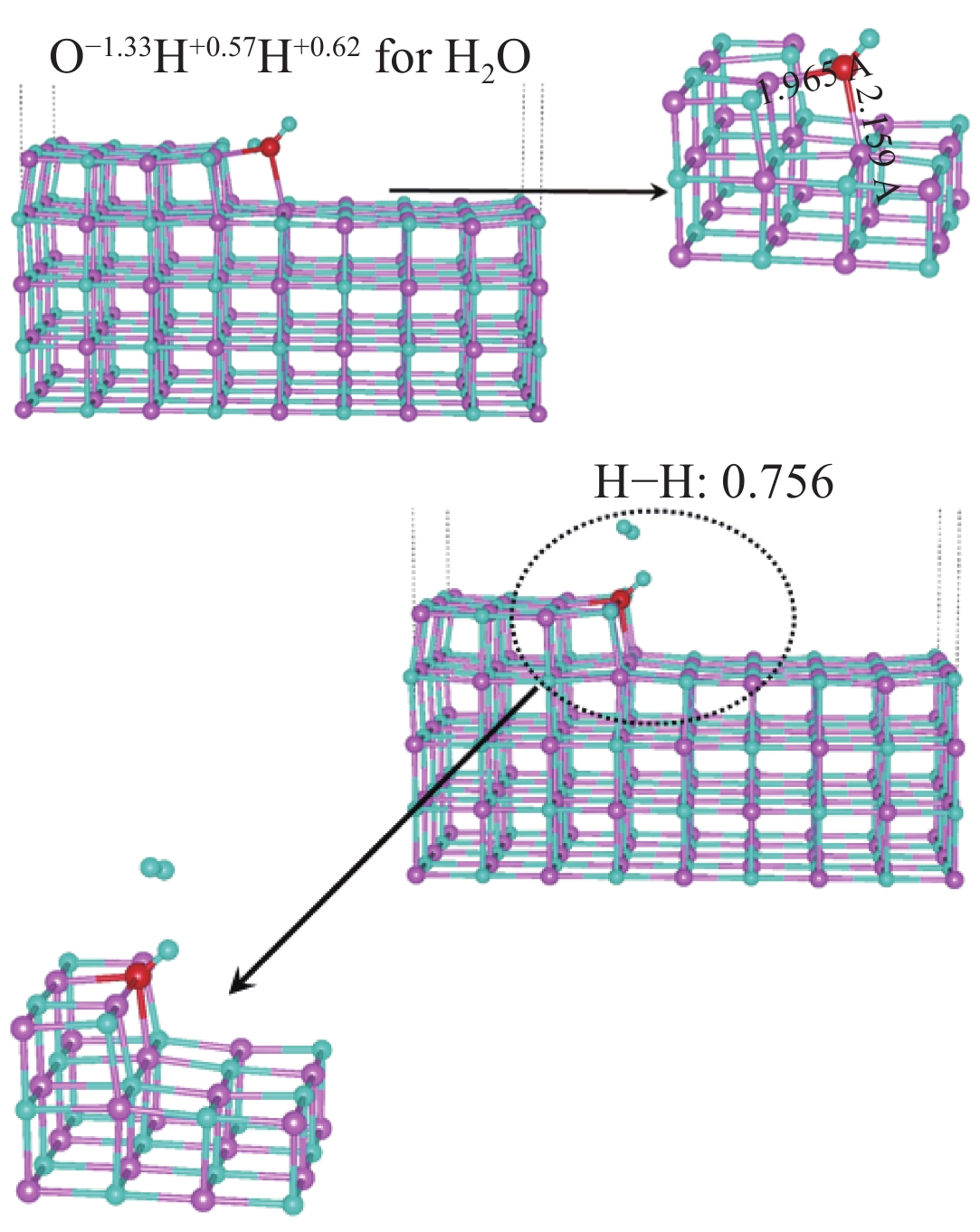
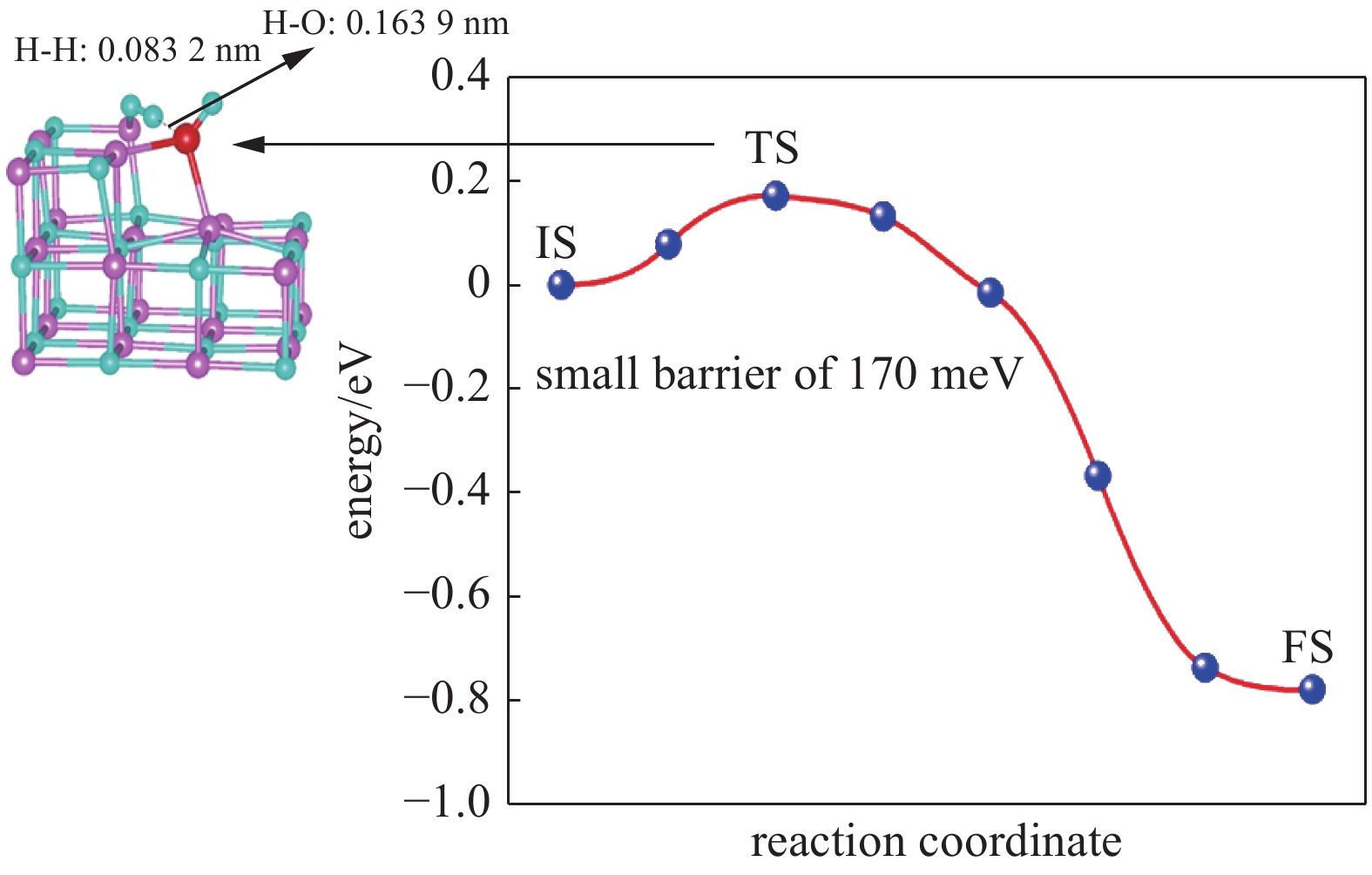
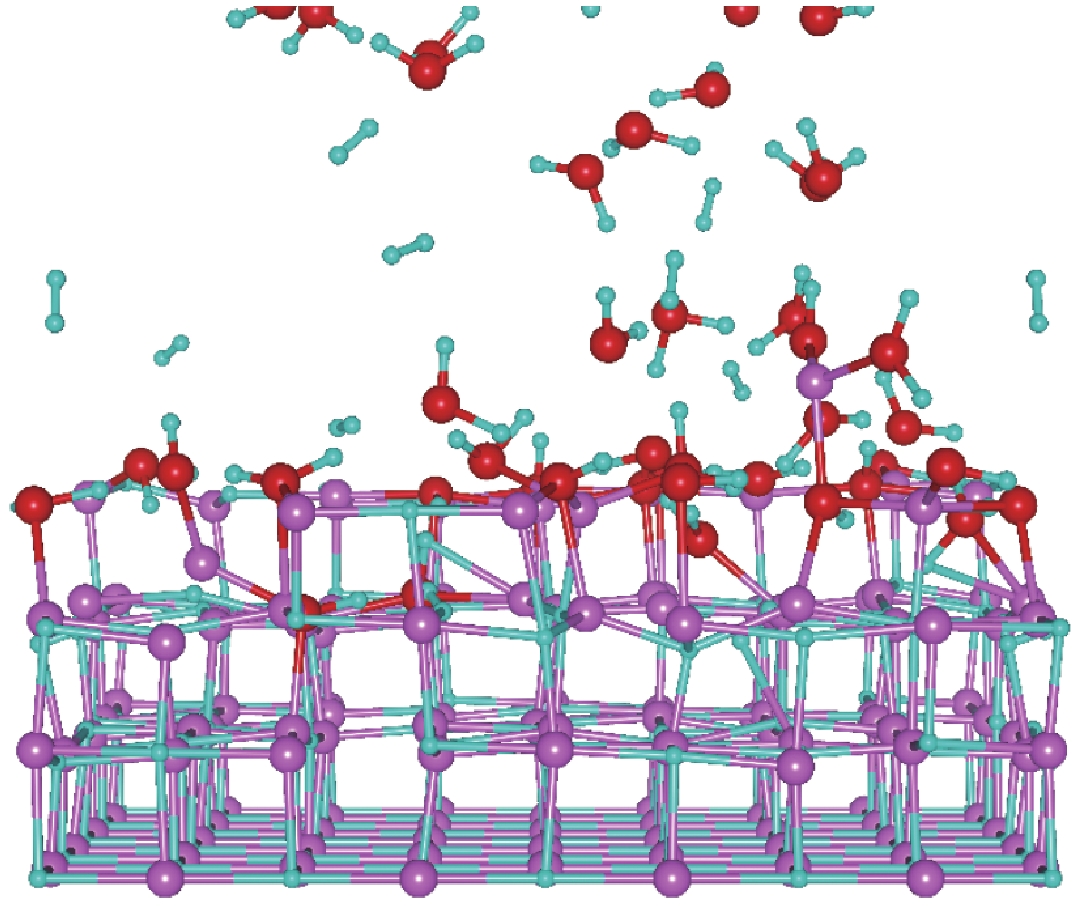
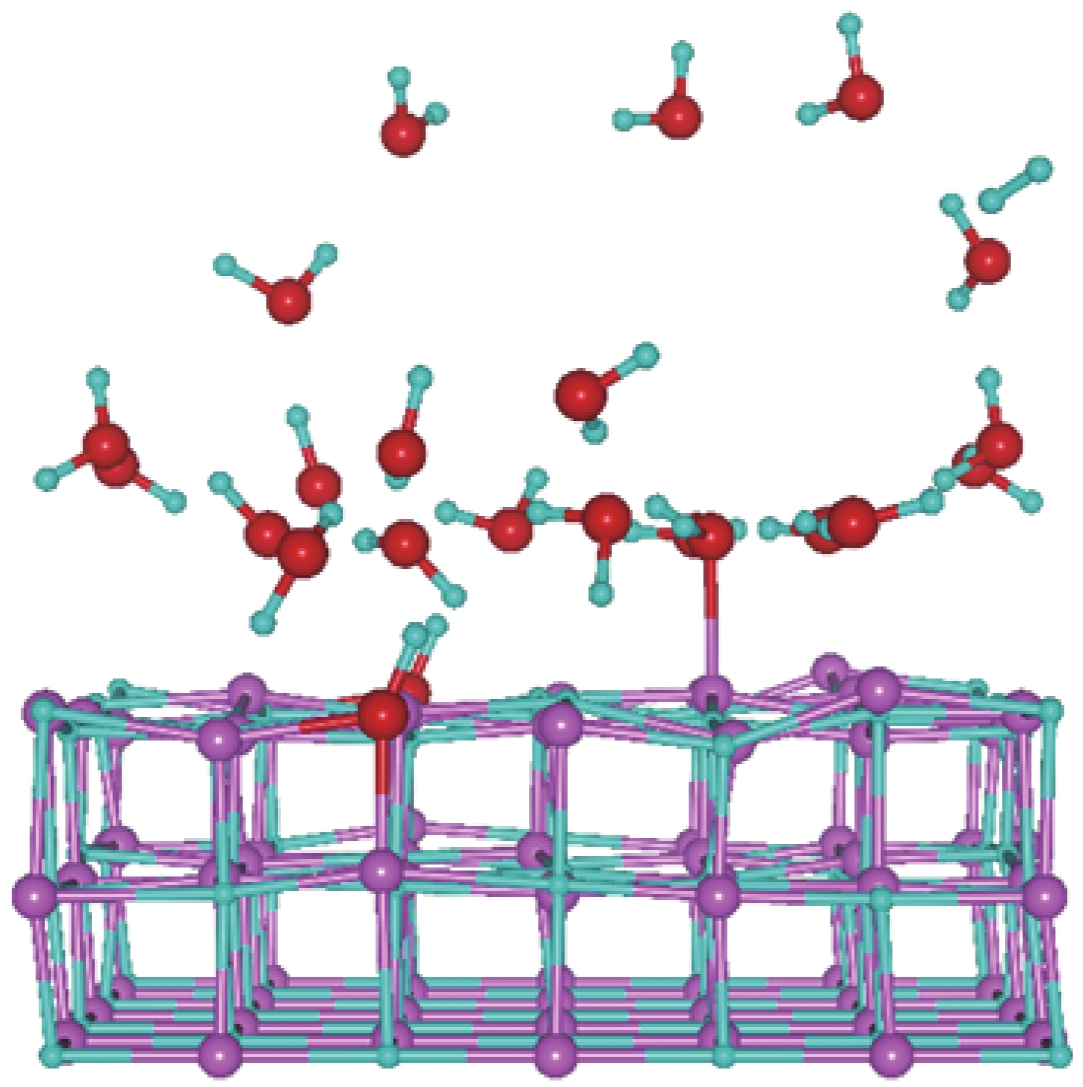
| [1] |
Haertling C, Hanrahan R J, Smith R. A literature review of reactions and kinetics of lithium hydride hydrolysis[J]. Journal of Nuclear Materials, 2006, 349(1/2): 195-233.
|
| [2] |
Chen P, Xiong Z, Luo J, et al. Interaction between lithium amide and lithium hydride[J]. Journal of Physical Chemistry B, 2003, 107(39): 10967-10970. doi: 10.1021/jp034149j
|
| [3] |
Kojima Y, Kawai Y, Kimbara M, et al. Hydrogen generation by hydrolysis reaction of lithium borohydride[J]. International Journal of Hydrogen Energy, 2004, 29(12): 1213-1217. doi: 10.1016/j.ijhydene.2003.12.009
|
| [4] |
Stober, K. J, Cantwell B J, Otaibi R A. Hypergolic ignition of lithium–aluminum–hydride-doped paraffin wax and nitric acid[J]. Journal of Propulsion and Power, 2020, 36(3): 435-445. doi: 10.2514/1.B37425
|
| [5] |
Houk K N, Rondan N G, Schleyer P V, et al. Transition structures for additions of lithium hydride and methyllithium to ethylene and acetylene[J]. Journal of the American Chemical Society, 1985, 107(9): 2821-2823. doi: 10.1021/ja00295a053
|
| [6] |
Kang X, Fang Z, Kong L, et al. Ammonia borane destabilized by lithium hydride: an advanced on-board hydrogen storage material[J]. Advanced Materials, 2008, 20(14): 2756-2759. doi: 10.1002/adma.200702958
|
| [7] |
Weber G, Sciora E, Guichard J, et al. New insight on the lithium hydride–water vapor reaction system[J]. International Journal of Hydrogen Energy, 2018, 43(50): 22557-22567. doi: 10.1016/j.ijhydene.2018.10.089
|
| [8] |
Dovesi R, Ermondi C, Ferrero E, et al. Hartree-Fock study of lithium hydride with the use of a polarizable basis set[J]. Physical Review B, 1984, 29(6): 3591-3600. doi: 10.1103/PhysRevB.29.3591
|
| [9] |
胡玉坤, 丁静, 彭晓峰, 等. 锂离子交换ZSM-5型分子筛中水分子吸附特性的分子模拟[J]. 硅酸盐学报, 2007(9):1247-1252. (Hu Yukun, Ding Jing, Peng Xiaofeng, et al. Molecular simultion of characteristics for water adsorption on ZSM-5 type zeolite doped by lithium ion[J]. Journal of the Chinese Ceramic Society, 2007(9): 1247-1252 doi: 10.3321/j.issn:0454-5648.2007.09.022
|
| [10] |
吕岩, 王涛, 马卫华. 基于密度泛函理论研究水在磷酸锂(100)表面的吸附[J]. 广州化工, 2016, 44(9):1-4. (Lü Yan, Wang Tao, Ma Weihua. Adsorption of water on Li3PO4 (100) surface from density functional theory[J]. Guangzhou Chemical Industry, 2016, 44(9): 1-4 doi: 10.3969/j.issn.1001-9677.2016.09.001
|
| [11] |
王林林, 朱灵燕, 刘跃龙, 等. 混合捕收剂在锂云母表面吸附行为的分子动力学模拟研究[J]. 有色金属(选矿部分), 2019(2):108-114. (Wang Linlin, Zhu Lingyan, Liu Yuelong, et al. Molecular dynamics simulation study on adsorption behavior of mixed collector on lithium mica surface[J]. Nonferrous Metals Mineral Processing Section, 2019(2): 108-114
|
| [12] |
李璐, 李奕, 郭欣, 等. 水在HfO2(111)和(110)表面的吸附与解离[J]. 物理化学学报, 2013(5):61-69. (Li Lu, Li Yi, Guo Xin, et al. Adsorption and dissociation of water on HfO2(111) and (110) surfaces[J]. Acta Physico-Chimica Sinica, 2013(5): 61-69
|
| [13] |
刘够生, 宋兴福, 汪瑾, 等. 水分子在MoO3原子簇模型表面吸附的密度泛函研究[J]. 分子催化, 2005, 19(2):136-140. (Liu Gousheng, Song Xingfu, Wang Jin, et al. Density functional theoretical study of water molecular adsorption on the surface of MoO3 with the cluster model[J]. Journal of Molecular Catalysis, 2005, 19(2): 136-140 doi: 10.3969/j.issn.1001-3555.2005.02.012
|
| [14] |
杜佳, 闵凡飞, 张明旭, 等. 水分子在铵伊利石表面吸附的密度泛函研究[J]. 中国矿业大学学报, 2017, 46(6):1349-1356. (Du Jia, Min Fanfei, Zhang Mingxu, et al. Mechanism of H2O adsorption on ammonium-illite surface based on density functional theory[J]. Journal of China University of Mining & Technology, 2017, 46(6): 1349-1356
|
| [15] |
薛严冰, 唐祯安, 孙伟民. 水分子在SnO2(110)表面吸附特性的密度泛函计算[J]. 大连交通大学学报, 2012, 33(5):93-96. (Xue Yanbing, Tang Zhen’an, Sun Weimin. Density functional calculation of properties of water molecule adsorption on SnO2 (110) surface[J]. Journal of Dalian Jiaotong University, 2012, 33(5): 93-96 doi: 10.3969/j.issn.1673-9590.2012.05.022
|
| [16] |
张瑶, 李照兵, 张鑫, 等. 水分子在Bi(111)表面上的吸附和自组装[J]. 中国科学(化学), 2016, 46(4):389-393. (Zhang Yao, Li Zhaobing, Zhang Xin, et al. Adsorption and self-assembly of water molecules on Bi (111)[J]. Scientia Sinica(Chimica), 2016, 46(4): 389-393
|
| [17] |
付纯鹤, 卢辉丽, 孙少瑞. 密度泛函理论方法研究6H-SiC(0001)表面对氧分子和水分子的吸附[J]. 化学物理学报, 2019(4):451-456. (Fu Chunhe, Lu Huili, Sun Shaorui. Density functional theory study for adsorption of oxygen and water molecules on 6H-SiC (0001) surface[J]. Chinese Journal of Chemical Physics, 2019(4): 451-456
|
| [18] |
赵红华, 江舒棋, 葛源源, 等. 不同阳离子基蒙脱石吸附水分子的分子动力学模拟分析[J]. 中国科学:技术科学, 2019, 49(6):703-715. (Zhao Honghua, Jiang Shuqi, Ge Yuanyuan, et al. Molecular dynamics simulation of water molecules adsorption by different cations based montmorillonite[J]. Scientia Sinica(Technologica), 2019, 49(6): 703-715
|
| [19] |
Oshikiri M, Boero M. Water molecule adsorption properties on the BiVO4 (100) surface[J]. Journal of Physical Chemistry B, 2006, 110(18): 9188-9194. doi: 10.1021/jp0555100
|
| [20] |
Kohtani M, Breaux G A, Jarrold M F, et al. Water molecule adsorption on protonated dipeptides[J]. Journal of the American Chemical Society, 2004, 126(4): 1206-1213. doi: 10.1021/ja0359557
|
| [21] |
Rangel E, Ruizchavarria G, Magana L F, et al. Water molecule adsorption on a titanium–graphene system with high metal coverage[J]. Carbon, 2009, 47(2): 531-533. doi: 10.1016/j.carbon.2008.11.037
|
| [22] |
Pang Z Q, Zhang Y, Rong Z, et al. Adsorption and dissociation of water on oxygen pre-covered Cu (110) observed with scanning tunneling microscopy[J]. Acta Physica Sinica, 2016, 65: 226801.
|
| [23] |
Wungu T D, Agusta M K, Saputro A G, et al. First principles calculation on the adsorption of water on lithium-montmorillonite (Li-MMT)[J]. Phys Condens Matter, 2012, 24: 475506. doi: 10.1088/0953-8984/24/47/475506
|
| [24] |
Wang C, Zhang K, Song P, et al. First-principles study of nitrogen adsorption and dissociation on PuH2 (111) surface[J]. Molecules, 2020, 25(8): 1891-1903. doi: 10.3390/molecules25081891
|




 下载:
下载: