Research progress on compatibility of ferritic/martensitic steel and austenitic stainless steel in static lead-bismuth eutectic environments
-
摘要:
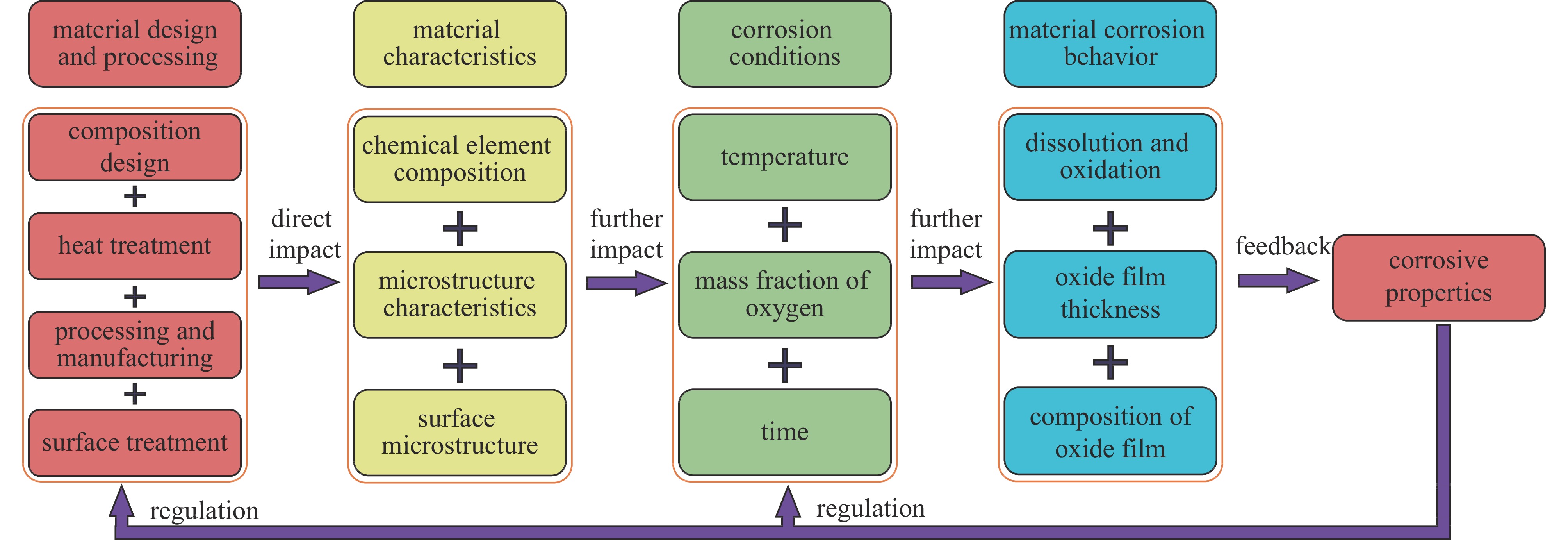
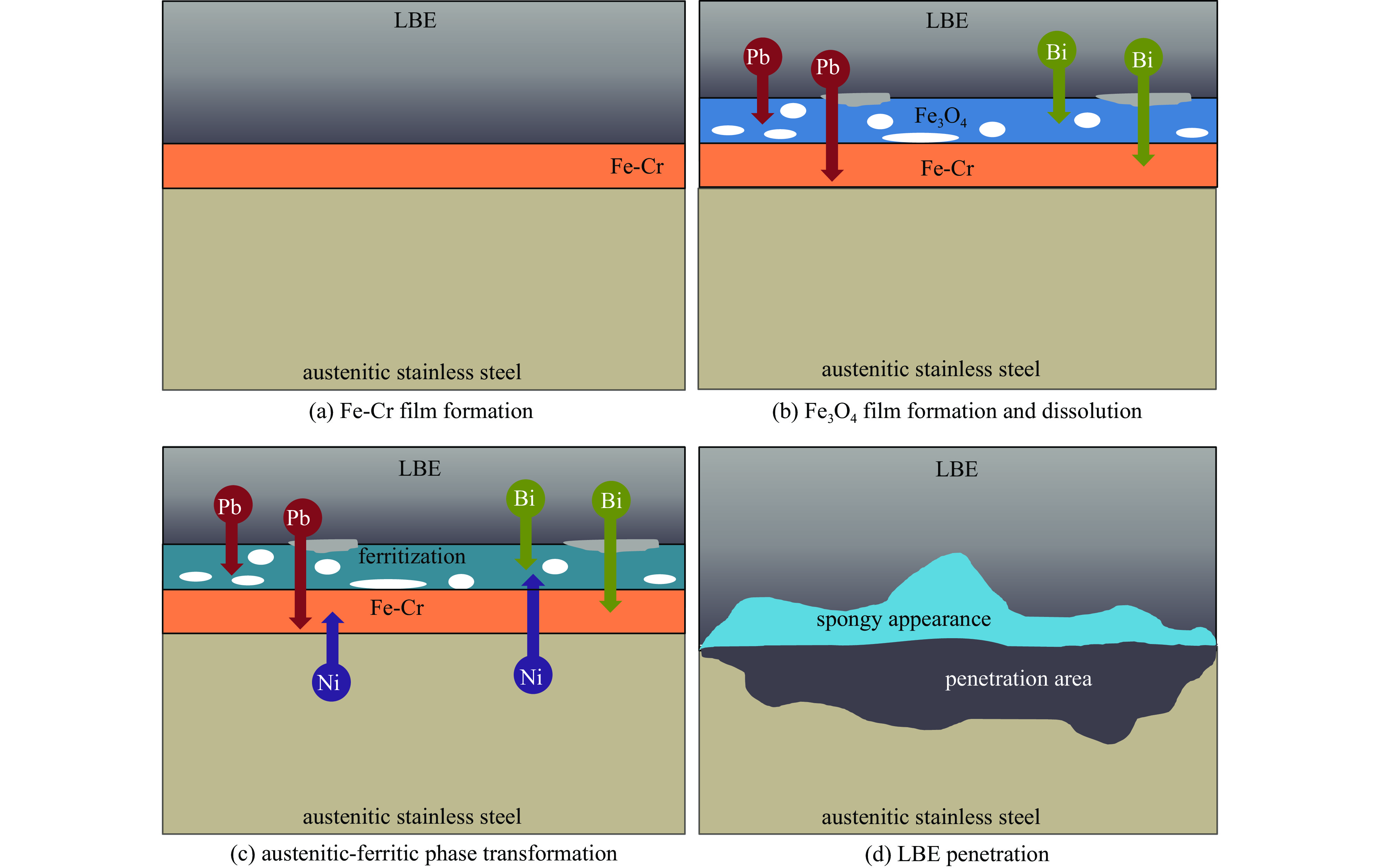
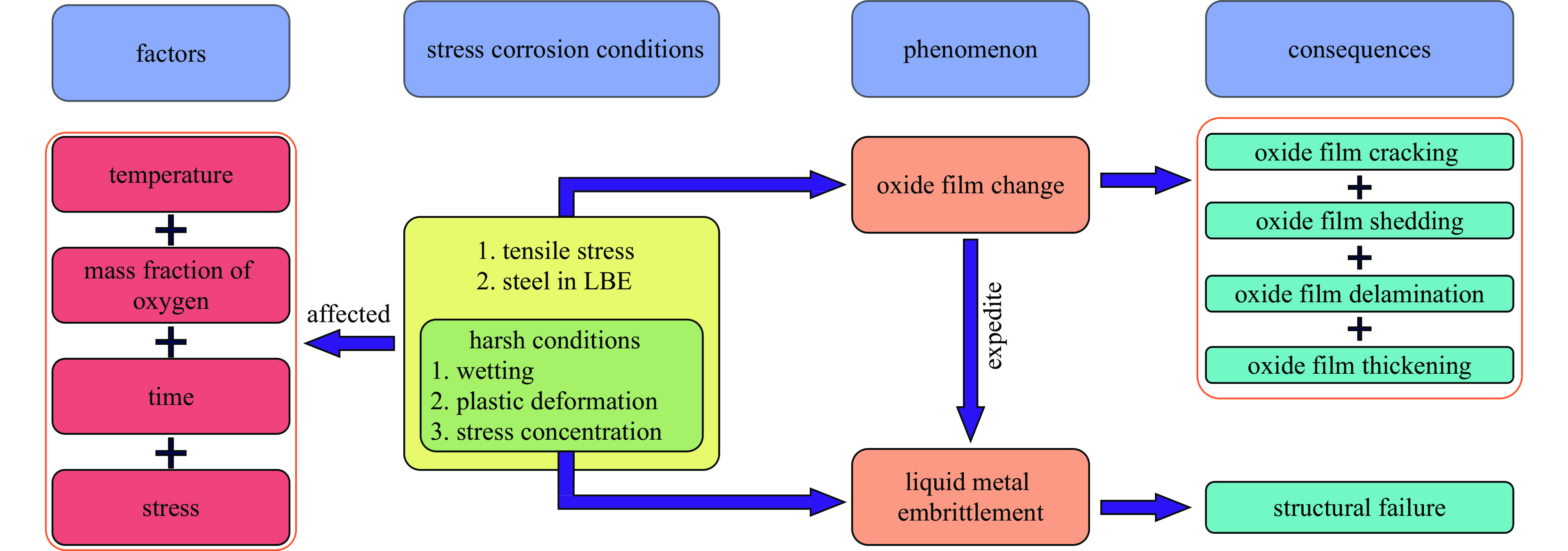
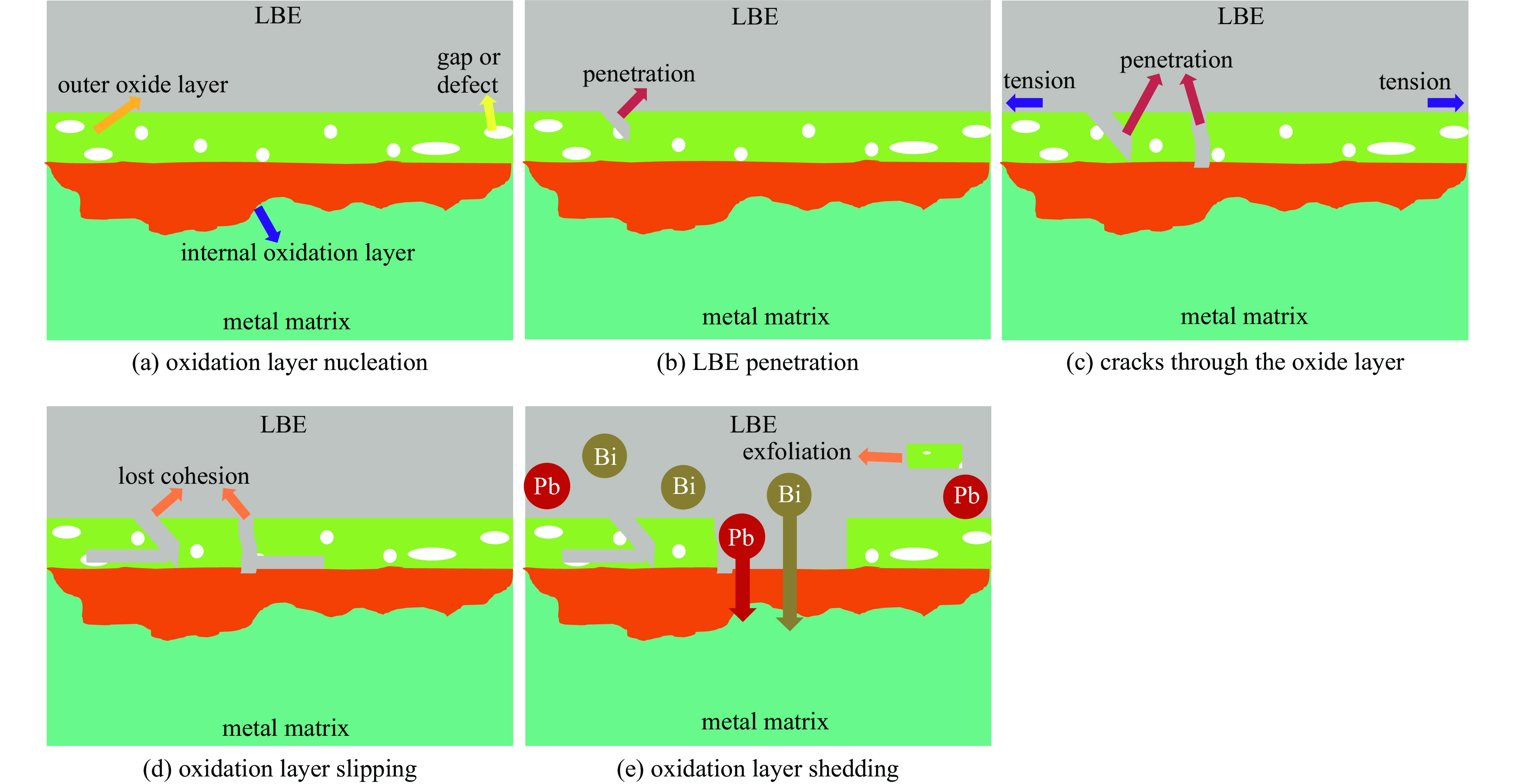
液态铅铋合金(LBE)是铅冷快中子反应堆(LFR)和加速器驱动次临界系统(ADS)的主要冷却剂材料。反应堆用结构材料(如铁素体/马氏体钢、奥氏体不锈钢等)在液态LBE环境下存在液态金属腐蚀(LMC)和应力腐蚀的问题,这些问题给钢结构材料的安全服役带来隐患。阐述了钢材铅铋腐蚀类型及机理,归纳了材料设计与处理(元素成分、热处理、加工制造和表面处理)和腐蚀条件(氧质量分数、腐蚀温度和腐蚀时间)对钢材铅铋腐蚀行为的影响机制;澄清了LBE环境下的应力腐蚀与金属脆化机制,总结了内外因素(材料种类、表面缺陷、热处理、氧质量分数、腐蚀温度和拉伸速率)对钢材拉伸性能的影响,并展望了未来铅铋反应堆结构材料的研究方向。建议面向未来的铅铋堆用钢应优化材料设计和处理方式(提高Si、Al等元素的含量、表面镀膜和热处理)同时控制LBE中环境参数(温度、氧质量分数和腐蚀时间)以提高钢材的耐铅铋腐蚀性能。
Abstract:The lead-bismuth eutectic (LBE) is one of the most promising coolants for the lead-cooled fast reactor (LFR) and the accelerator driven sub-critical system (ADS). The liquid metal corrosion (LMC) and stress corrosion in liquid LBE environments are inevitable critical issues for structural materials such as ferritic/martensitic steel, austenitic stainless steel, which are potential safety hazards for the service of structural materials. In this work, the corrosion types and liquid metal embrittlement (LME) mechanism of steel are illustrated, the influences of material design and processing (chemical composition, heat treatment, processing and manufacturing and surface treatment) and corrosion conditions (temperature, mass fraction of oxygen and time) on the corrosion behavior and of steels are summarized. In addition, stress corrosion and LME in liquid LBE is clarified, and the effects of internal and external factors (types, surface defect, heat treatment, mass fraction of oxygen, corrosion temperature and tensile rates) on the mechanical properties of steels are analyzed. In the end, the future research interests of steels in LBE are prospected. The future steel used in liquid LBE is proposed to optimize the material design and treatment (appropriately increasing the content of Si and Al, surface coating and heat treatment) and control the environmental parameters (temperature, mass fraction of oxygen and corrosion time) in LBE to improve its corrosion resistance.
-
Key words:
- steel /
- liquid lead-bismuth eutectic alloy /
- compatibility /
- corrosion mechanism /
- stress corrosion
-
-
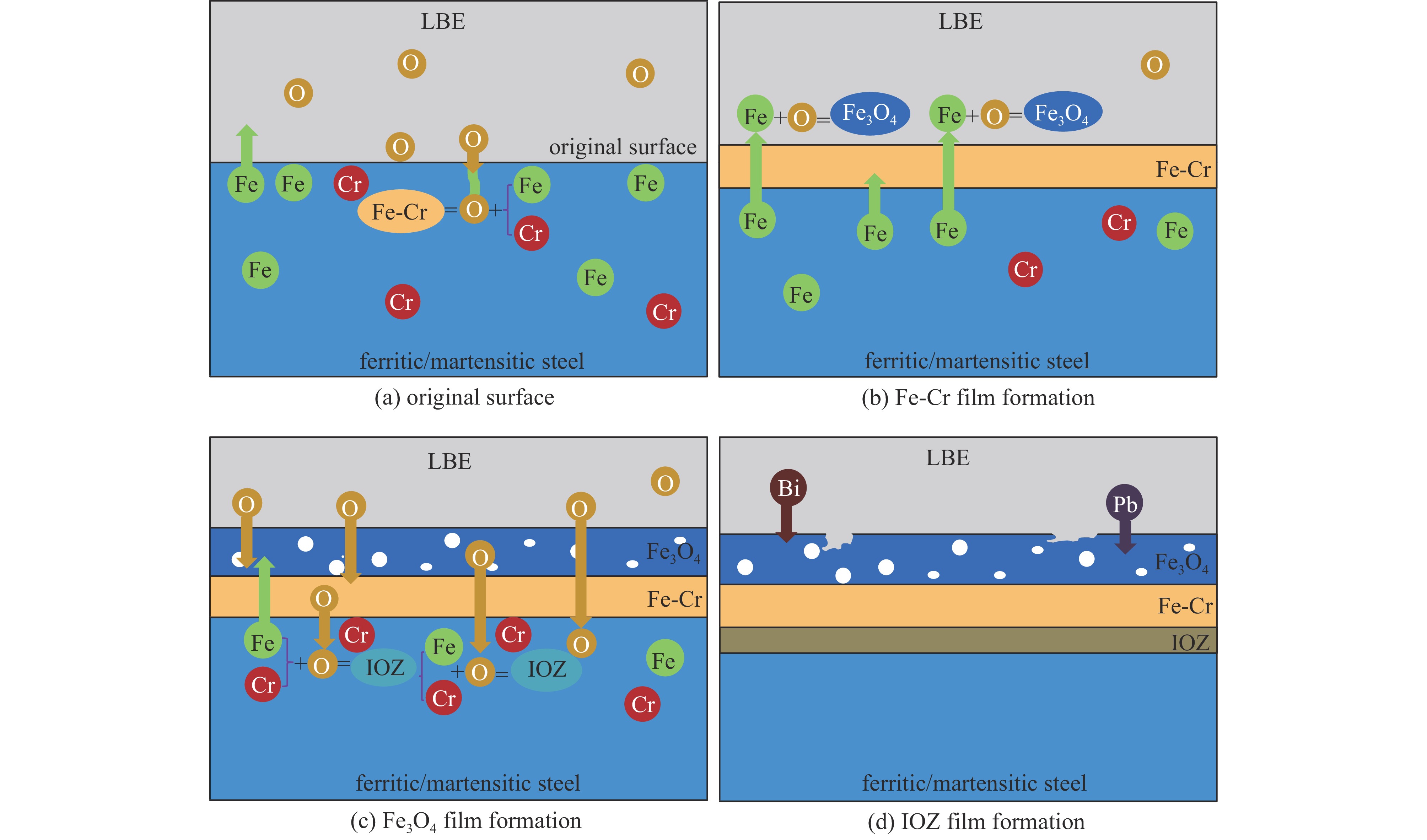
[1] 李琼, 刘紫静, 肖豪, 等. 基于Kriging代理模型的铅铋反应堆智能优化方法[J]. 强激光与粒子束, 2022, 34:056007 doi: 10.11884/HPLPB202234.210560Li Qiong, Liu Zijing, Xiao Hao, et al. Intelligent optimization method for lead-bismuth reactor based on Kriging surrogate model[J]. High Power Laser and Particle Beams, 2022, 34: 056007 doi: 10.11884/HPLPB202234.210560 [2] 方海涛, 赵永松, 张喜林, 等. 铅冷快堆M2LFR-1000堆芯燃料管理方案设计[J]. 强激光与粒子束, 2018, 30:096003 doi: 10.11884/HPLPB201830.180083Fang Haitao, Zhao Yongsong, Zhang Xilin, et al. In-core fuel management strategy design of lead-cooled fast reactor M2LFR-1000[J]. High Power Laser and Particle Beams, 2018, 30: 096003 doi: 10.11884/HPLPB201830.180083 [3] 吴宜灿, FDS团队. 第四代核能系统铅基反应堆前景展望[J]. 科技导报, 2015, 33(14):12Wu Yichan, FDS Team. Prospects of lead-based reactors for fourth-generation nuclear energy systems[J]. Science & Technology Review, 2015, 33(14): 12 [4] 张亮, 孙胜, 孙寿华, 等. Dragon程序在金属燃料铅铋快堆堆芯计算中的应用与偏差分析[J]. 强激光与粒子束, 2022, 34:056005 doi: 10.11884/HPLPB202234.220001Zhang Liang, Sun Sheng, Sun Shouhua, et al. Preliminary application of neutronics calculation in LFR reactor with metallic fuel using Dragon code[J]. High Power Laser and Particle Beams, 2022, 34: 056005 doi: 10.11884/HPLPB202234.220001 [5] Zhang Jinsuo, Li Ning. Review of the studies on fundamental issues in LBE corrosion[J]. Journal of Nuclear Materials, 2008, 373(1/3): 351-377. [6] Zhang Jinsuo. A review of steel corrosion by liquid lead and lead–bismuth[J]. Corrosion Science, 2009, 51(6): 1207-1227. doi: 10.1016/j.corsci.2009.03.013 [7] Yamaki E, Ginestar K, Martinelli L. Dissolution mechanism of 316L in lead–bismuth eutectic at 500 ℃[J]. Corrosion Science, 2011, 53(10): 3075-3085. doi: 10.1016/j.corsci.2011.05.031 [8] 阮章顺, 秦博, 付晓刚, 等. 燃料元件包壳材料CN-1515不锈钢在可控氧铅铋环境下的腐蚀行为[J]. 原子能科学技术, 2021, 55(5):901-908Ruan Zhangshun, Qin Bo, Fu Xiaogang, et al. Corrosion behavior of fuel cladding material CN-1515 stainless steel in lead-bismuth eutectic alloy with oxygen control[J]. Atomic Energy Science and Technology, 2021, 55(5): 901-908 [9] 胡亚东. T91钢在静态铅铋氧控环境中应力腐蚀行为研究[D]. 合肥: 中国科学技术大学, 2018Hu Yadong. Stress corrosion behavior of T91 steel in static lead-bismuth eutectic with oxygen control[D]. Hefei: University of Science and Technology of China, 2018 [10] Serena B, Sebastiano C, Carlo C, et al. Material performance in lead and lead-bismuth alloy[J]. Reference Module in Materials Science and Materials Engineering, 2012, 4: 218-241. [11] 李明扬. T91和316L钢在铅铋合金中的腐蚀行为研究[D]. 合肥: 合肥工业大学, 2014Li Mingyang. Study on the corrosion behavior of T91 and 316L steels in liquid LBE[D]. Hefei: Hefei University of Technology, 2014 [12] Lambrinou K, Charalampopoulou E, Van Der Donck T, et al. Dissolution corrosion of 316L austenitic stainless steels in contact with static liquid lead-bismuth eutectic (LBE) at 500 ℃[J]. Journal of Nuclear Materials, 2017, 490: 9-27. doi: 10.1016/j.jnucmat.2017.04.004 [13] Schroer, C, Konys J. Physical chemistry of corrosion and oxygen control in liquid lead and lead-bismuth eutectic[R]. FZKA 7364, 2007. [14] 龚星, 肖军, 王浩, 等. 铁素体/马氏体钢和奥氏体不锈钢的液态铅铋腐蚀行为与机理[J]. 核科学与工程, 2020, 40(5):864-871Gong Xing, Xiao Jun, Wang Hao, et al. Corrosion behavior and mechanisms of ferritic/martensitic steels and austenitic stainless steels in liquid lead-bismuth eutectic[J]. Nuclear Science and Engineering, 2020, 40(5): 864-871 [15] 田书建, 张建武. 316L和T91不锈钢在550 ℃静态铅铋合金中的腐蚀行为[J]. 中国科学技术大学学报, 2015, 45(9):751-756Tian Shujian, Zhang Jianwu. Corrosion behavior of 316L and T91 steels in stagnant lead-bismuth eutectic at 550 ℃[J]. Journal of University of Science and Technology of China, 2015, 45(9): 751-756 [16] Kurata Y, Futakawa M, Saito S. Corrosion behavior of steels in liquid lead–bismuth with low oxygen concentrations[J]. Journal of Nuclear Materials, 2008, 373(1/3): 164-178. [17] Hosemann P, Frazer D, Stergar E, et al. Twin boundary-accelerated ferritization of austenitic stainless steels in liquid lead–bismuth eutectic[J]. Scripta Materialia, 2016, 118: 37-40. doi: 10.1016/j.scriptamat.2016.02.029 [18] Tsisar V, Schroer C, Wedemeyer O, et al. Characterization of corrosion phenomena and kinetics on T91 ferritic/martensitic steel exposed at 450 and 550 ℃ to flowing Pb-Bi eutectic with 10-7 mass% dissolved oxygen[J]. Journal of Nuclear Materials, 2017, 494: 422-438. doi: 10.1016/j.jnucmat.2017.07.031 [19] 李君瑜, 周立军. 国内外铅铋氧控研究现状[J]. 化工管理, 2022(5):52-54 doi: 10.19900/j.cnki.ISSN1008-4800.2022.05.017Li Junyu, Zhou Lijun. Status of oxygen control research on lead-bismuth alloy at home and abroad[J]. Chemical Enterprise Management, 2022(5): 52-54 doi: 10.19900/j.cnki.ISSN1008-4800.2022.05.017 [20] Müller G, Schumacher G, Zimmermann F. Investigation on oxygen controlled liquid lead corrosion of surface treated steels[J]. Journal of Nuclear Materials, 2000, 278(1): 85-95. doi: 10.1016/S0022-3115(99)00211-1 [21] Martinelli L, Balbaud-Célérier F, Terlain A, et al. Oxidation mechanism of an Fe–9Cr–1Mo steel by liquid Pb–Bi eutectic alloy at 470 ℃ (Part II)[J]. Corrosion Science, 2008, 50(9): 2537-2548. doi: 10.1016/j.corsci.2008.06.051 [22] Roy M, Martinelli L, Ginestar K, et al. Dissolution and oxidation behaviour of various austenitic steels and Ni rich alloys in lead-bismuth eutectic at 520 ℃[J]. Journal of Nuclear Materials, 2016, 468: 153-163. doi: 10.1016/j.jnucmat.2015.11.005 [23] Tsisar V, Stergar E, Gavrilov S, et al. Effect of variation in oxygen concentration in static Pb–Bi eutectic on long-term corrosion performance of Al-alloyed austenitic steels at 500 ℃[J]. Corrosion Science, 2022, 195: 109963. doi: 10.1016/j.corsci.2021.109963 [24] Kurata Y. Corrosion behavior of Si-enriched steels for nuclear applications in liquid lead–bismuth[J]. Journal of Nuclear Materials, 2013, 437(1/3): 401-408. [25] Kurata Y, Futakawa M, Saito S. Comparison of the corrosion behavior of austenitic and ferritic/martensitic steels exposed to static liquid Pb–Bi at 450 and 550 ℃[J]. Journal of Nuclear Materials, 2005, 343(1): 333-340. [26] 董红. 铅铋共晶合金与T91钢的相容性[D]. 沈阳: 沈阳理工大学, 2013Dong Hong. The compatibility of LBE alloy and T91 steel[D]. Shenyang: Shenyang Ligong University, 2013 [27] Charalampopoulou E, Lambrinou K, Van der Donck T, et al. Early stages of dissolution corrosion in 316L and DIN 1.4970 austenitic stainless steels with and without anticorrosion coatings in static liquid lead-bismuth eutectic (LBE) at 500° C[J]. Materials Characterization, 2021, 178: 111234. doi: 10.1016/j.matchar.2021.111234 [28] Wang J, Rong L J, Li D Z, et al. Effect of welding thermal cycles on the oxidation resistance of 9 wt. % Cr heat resistant steels in 550 ℃ lead-bismuth eutectic[J]. Applied Surface Science, 2016, 389: 930-941. doi: 10.1016/j.apsusc.2016.08.042 [29] Johnson A L, Parsons D, Manzerova J, et al. Spectroscopic and microscopic investigation of the corrosion of 316/316L stainless steel by lead–bismuth eutectic (LBE) at elevated temperatures: importance of surface preparation[J]. Journal of Nuclear Materials, 2004, 328(2/3): 88-96. [30] Martín-Muñoz F J, Soler-Crespo L, Gómez-Briceño D. Assessment of the influence of surface finishing and weld joints on the corrosion/oxidation behaviour of stainless steels in lead bismuth eutectic[J]. Journal of Nuclear Materials, 2011, 416(1/2): 80-86. [31] 丁祥彬, 罗梦, 路广遥, 等. 不同表面处理对316L钢焊缝耐液态铅铋腐蚀的影响[J]. 焊接, 2019(2):21-25Ding Xiangbin, Luo Meng, Lu Guangyao, et al. Effect of different surface treatment on 316L steel welds in the liquid lead-bismuth alloy[J]. Welding & Joining, 2019(2): 21-25 [32] Engelko V, Mueller G, Rusanov A, et al. Surface modification/alloying using intense pulsed electron beam as a tool for improving the corrosion resistance of steels exposed to heavy liquid metals[J]. Journal of Nuclear Materials, 2011, 415(3): 270-275. doi: 10.1016/j.jnucmat.2011.04.030 [33] 柏佩文. 15-15Ti钢上SiC薄膜的制备及其耐铅铋合金腐蚀性能的研究[D]. 合肥: 合肥工业大学, 2017Bai Peiwen. The preparation and corrosion resistance in Pb-Bi alloy of SiC films on 15-15Ti steel[D]. Hefei: Hefei University of Technology, 2017 [34] Ma Zhiwei, Shen Tielong, Wang Zhiguang, et al. Improving the oxidation resistance of SIMP steel to liquid Pb-Bi eutectic by shot peening treatments[J]. Applied Surface Science, 2022, 578: 151910. doi: 10.1016/j.apsusc.2021.151910 [35] Balbaud-Celerier F, Deloffre P, Terlain A, et al. Corrosion of metallic materials in flowing liquid lead-bismuth[J]. Journal de Physique IV, 2002, 12(8): 177-190. [36] 田书建. T91和15-15Ti钢在500 ℃液态铅铋合金氧控条件下腐蚀行为与机理研究[D]. 合肥: 中国科学技术大学, 2016Tian Shujian. Corrosion behavior and mechanism of T91 and 15-15Ti steels in liquid lead-bismuth eutectic under oxygen control at 500 ℃[D]. Hefei: University of Science and Technology of China, 2016 [37] Sapundjiev D, Van Dyck S, Bogaerts W. Liquid metal corrosion of T91 and A316L materials in Pb–Bi eutectic at temperatures 400-600 ℃[J]. Corrosion Science, 2006, 48(3): 577-594. doi: 10.1016/j.corsci.2005.04.001 [38] Benamati G, Fazio C, Piankova H, et al. Temperature effect on the corrosion mechanism of austenitic and martensitic steels in lead–bismuth[J]. Journal of Nuclear Materials, 2002, 301(1): 23-27. doi: 10.1016/S0022-3115(01)00723-1 [39] Gnecco F, Ricci E, Bottino C, et al. Corrosion behaviour of steels in lead–bismuth at 823 K[J]. Journal of Nuclear Materials, 2004, 335(2): 185-188. doi: 10.1016/j.jnucmat.2004.07.013 [40] Cionea C, Abad M D, Aussat Y, et al. Oxide scale formation on 316L and FeCrAl steels exposed to oxygen controlled static LBE at temperatures up to 800 ℃[J]. Solar Energy Materials and Solar Cells, 2016, 144(11): 235-246. [41] Yeliseyeva O, Tsisar V, Benamati G. Influence of temperature on the interaction mode of T91 and AISI 316L steels with Pb–Bi melt saturated by oxygen[J]. Corrosion Science, 2008, 50(6): 1672-1683. doi: 10.1016/j.corsci.2008.02.006 [42] Martinelli L, Balbaud-Célérier F, Picard G, et al. Oxidation mechanism of a Fe–9Cr–1Mo steel by liquid Pb–Bi eutectic alloy (Part III)[J]. Corrosion Science, 2008, 50(9): 2549-2559. doi: 10.1016/j.corsci.2008.06.049 [43] Van den Bosch J, Sapundjiev D, Almazouzi A. Effects of temperature and strain rate on the mechanical properties of T91 material tested in liquid lead bismuth eutectic[J]. Journal of Nuclear Materials, 2006, 356(1/3): 237-246. [44] Fazio C, Benamati G, Martini C, et al. Compatibility tests on steels in molten lead and lead–bismuth[J]. Journal of Nuclear Materials, 2001, 296(1/3): 243-248. [45] Müller G, Heinzel A, Konys J, et al. Results of steel corrosion tests in flowing liquid Pb/Bi at 420-600 ℃ after 2000 h[J]. Journal of Nuclear Materials, 2002, 301(1): 40-46. doi: 10.1016/S0022-3115(01)00725-5 [46] 田书建, 姜志忠, 张敏, 等. T91钢在氧浓度为0.01 ppm静态铅铋合金中的界面腐蚀特征[J]. 原子能科学技术, 2017, 51(1):158-164 doi: 10.7538/yzk.2017.51.01.0158Tian Shujian, Jiang Zhizhong, Zhang Min, et al. Interface corrosion characteristics of T91 steel in static lead-bismuth eutectic with 0.01 ppm oxygen[J]. Atomic Energy Science and Technology, 2017, 51(1): 158-164 doi: 10.7538/yzk.2017.51.01.0158 [47] 刘静. T91和316L钢在液态Pb-Bi共晶合金中应力腐蚀行为研究[D]. 合肥: 中国科学技术大学, 2015Liu Jing. Stress corrosion behavior of T91 and 316L steels in liquid lead-bismuth eutectic[D]. Hefei: University of Science and Technology of China, 2015 [48] OECD, Nuclear Energy Agency. Handbook on lead-bismuth eutectic alloy and lead properties, materials compatibility, thermal-hydraulics and technologies[M]. Nuclear Energy Agency, 2015. [49] Hamouche-Hadjem Z, Auger T, Guillot I, et al. Susceptibility to LME of 316L and T91 steels by LBE effect of strain rate[J]. Journal of Nuclear Materials, 2008, 376(3): 317-321. doi: 10.1016/j.jnucmat.2008.02.031 [50] Liu J, Jiang Z Z, Tian S J, et al. Stress corrosion behavior of T91 steel in static lead–bismuth eutectic at 480 ℃[J]. Journal of Nuclear Materials, 2016, 468: 299-304. doi: 10.1016/j.jnucmat.2015.09.032 [51] Luo L, Jiang Z Z, Xiao Z Q, et al. Cracking and exfoliation behavior of oxide scale on T91 steel under different tensile stresses in oxygen-controlled lead-bismuth eutectic at 550 ℃[J]. Corrosion Science, 2021, 183: 109324. doi: 10.1016/j.corsci.2021.109324 [52] Van Den Bosch J, Coen G, Hosemann P, et al. On the LME susceptibility of Si enriched steels[J]. Journal of Nuclear Materials, 2012, 429(1/3): 105-112. [53] Gorse D, Auger T, Vogt J B, et al. Influence of liquid lead and lead–bismuth eutectic on tensile, fatigue and creep properties of ferritic/martensitic and austenitic steels for transmutation systems[J]. Journal of Nuclear Materials, 2011, 415(3): 284-292. doi: 10.1016/j.jnucmat.2011.04.047 [54] Stergar E, Eremin S G, Gavrilov S, et al. Influence of LBE long term exposure and simultaneous fast neutron irradiation on the mechanical properties of T91 and 316L[J]. Journal of Nuclear Materials, 2016, 473: 28-34. doi: 10.1016/j.jnucmat.2016.02.008 [55] Long B, Tong Zhenwei, Gröschel F, et al. Liquid Pb–Bi embrittlement effects on the T91 steel after different heat treatments[J]. Journal of Nuclear Materials, 2008, 377(1): 219-224. doi: 10.1016/j.jnucmat.2008.02.050 [56] Liu Jian, Yan Wei, Sha Wei, et al. Effects of temperature and strain rate on the tensile behaviors of SIMP steel in static lead bismuth eutectic[J]. Journal of Nuclear Materials, 2016, 473: 189-196. doi: 10.1016/j.jnucmat.2016.02.032 [57] Dai Yong, Long B, Groeschel F. Slow strain rate tensile tests on T91 in static lead–bismuth eutectic[J]. Journal of Nuclear Materials, 2006, 356(1/3): 222-228. -




 下载:
下载: