Preparation and electrochemical performances of ternary LiNi1-x-yCoxMnyO2 cathode material
-
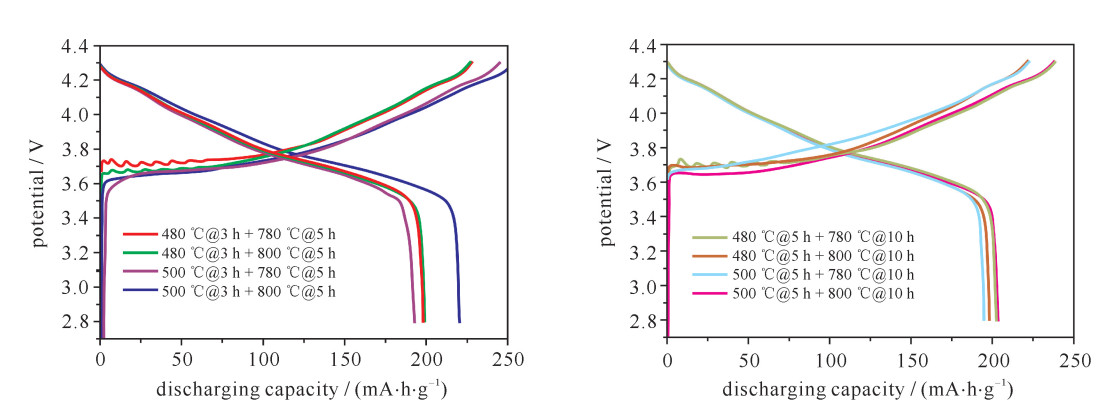
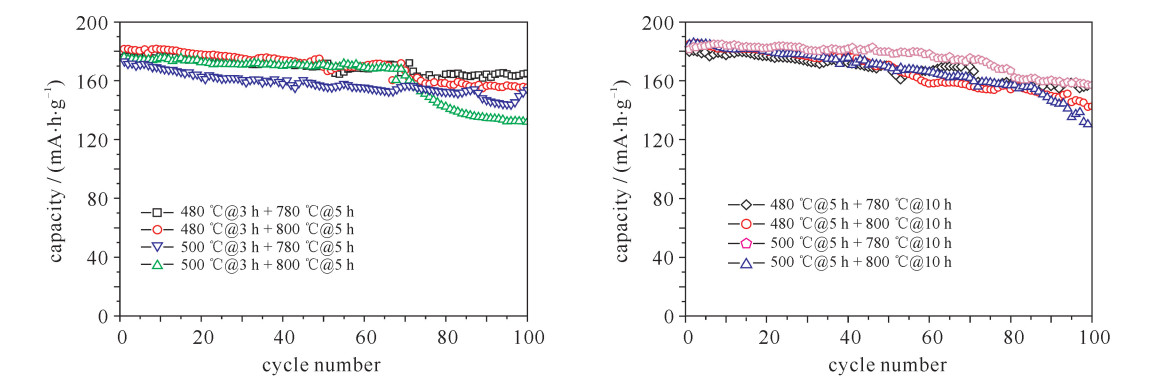
摘要: LiNi1-x-yCoxMnyO2正极材料作为最有商业化前途的锂离子电池正极材料,近年来成为研究者关注的焦点。但目前针对该材料合成工艺的研究还较少。对LiNi0.8Co0.15Mn0.05O2开展了不同的烧结工艺研究,并对制备出的正极材料进行了表征和性能测试。研究发现在0.1C (电池容量额定值)倍率下充放电比容量为200 mA·h·g-1左右,在1C倍率循环100次下,480 ℃@3 h + 780 ℃@5 h和500 ℃@5 h + 780 ℃@10 h两种烧结工艺下容量保持率分别为94%和86%,说明用这两种工艺制备的正极材料的综合性能最优。Abstract: As the most promising commercial lithium-ion battery, the LiNi1-x-yCoxMnyO2 cathode have become the focus of researchers in recent years. However, there are less researches on the synthetic process of this material. This paper discusses various sintering processes of LiNi0.8Co0.15Mn0.05O2, and the characterization and electrochemical performances testing of the prepared cathode materials. The research shows that the material has higher specific capacity (about 200 mA·h·g-1) at 0.1C (C is battery capacity rating value) charging-discharging rate. At 1C and 100 times of charging-discharging, the capacity retention ratios of 480 ℃@3 h + 780 ℃@5 h and 500 ℃@5 h + 780 ℃@10 h sintering processes are 94% and 86%, respectively, which illustrates the synthetical properties of the cathode materials prepared by these two processes are optimal.
-
脉冲磁场的测量是电磁脉冲研究领域的重点之一[1-6]。目前,脉冲磁场传感器的发展迅速,其中,屏蔽小环磁场传感器因其结构简单、性能可靠,特别是对于中低频信号的测量精度较高而备受关注。但是磁偶极子天线的输出端结构破坏了天线的对称性由于磁偶极子天线不可避免地存在输出端结构,破坏了天线的对称性,因此对于多极性的电磁脉冲环境测量通常很难满足测量要求,极易被径向电场干扰导致磁场波形失真[7]。尤其是对雷电回击、高空核爆的近场磁场测量,现有的脉冲磁场传感器很难避免电场干扰[8]。因此,本文研制了一种基于双环差分结构的脉冲磁场传感器,通过构造差分结构可以去除绝大部分径向电场响应分量,从而获得了较为纯净的磁场响应,实现了对雷电近场环境的准确测量。
1. 双环差分测量原理
在电气小条件下,磁偶极子天线可由激励源和等效的集总电感表示,因此单环磁场传感器的等效电路如图1所示[9]。其中L为磁偶极子的等效集总电感,ZL为负载阻抗,ω为测得的磁场信号的频率,Bi为入射磁场的磁感应强度。小环天线将被拾取磁场强度部分作为激励源,则磁场响应的大小和方向取决于入射磁场和传感器的灵敏度。此时传感器的灵敏度可以用等效面积Ae表示。激励源具有电压量纲,其拾取量可以表示为
∂Bi∂tAe 。则负载电压VL可以表示为
V(jω)=jωAeZLjωL+ZLB(jω) (1) 由式(1)可知,根据磁偶极子天线的工作频率范围,磁场传感器可以分为微分模式,过渡模式和自积分模式[10]。当ZL
≫ ωL,单环传感器的负载电压VL与微分磁场成正比,可表示为VL=Ae⋅∂∂tB (2) 感应近场条件下,交变电场和交变磁场在相位上正交,因而在磁场测量中极易受到电场干扰。在屏蔽小环天线的基础上引入双环差分结构,即将两个相同的屏蔽小环天线平行镜像对称放置,如图2所示。同时将增大环天线的匹配阻抗从而减小环感应电流,以降低环间互扰带来的不利影响。其中,E,H分别表示入射的脉冲电场和脉冲磁场分量,用下标1和2对双环进行区分,并将其输出端电压U按照贡献来源不同分为UE和UH,分别表示电场和磁场响应的贡献。
根据电磁感应原理,则有
{U1=UE1+UH1U2=UE2+UH2 (3) 两个开路截断的屏蔽小环天线镜像对称放置时,由于轴向磁通量反向而径向电通量相同,则双环天线的磁场响应电压分量应反相,而电场响应分量方向相同。则有
U1−U2=UH1−UH2=2UH (4) 联立式(2),(4)可知,差分后的输出电压即为的微分磁场与天线等效面积乘积的2倍,且并不包含电场响应分量。
2. 双环式差分磁场传感器的设计与实现
基于双环差分测量原理,本文提出一种脉冲磁场传感器如图3所示,图3(a)为工作原理图,图3(b)为实物图。传感器主要由双环天线、光传输系统组成,光传输系统又由光发射机、光纤和光接收机组成。双环天线的输出端分别与光发射机的输入端连接,光发射机的输出端与光接收机的输入端通过光纤连接,光接收机的输出端接入示波器即可实现磁场测量。
由式(2)可知,环天线的输出电压与磁场微分成正比。为了得到可直接观测的磁场波形,每一个环天线的输出端串联了一个RC积分电路,在光发射机内完成电压信号的积分运算;其次,利用差分电路把两个积分磁场信号差分以去除电场响应分量,可以实现纯净磁场信号的输出。
2.1 双环天线
双环天线由两个屏蔽小环天线组成,主要负责将分布的磁场参量转换成终端电压。小环天线由一段弯成环形的半钢同轴电缆制成,左半环末端的内导体与右半环屏蔽层短路焊接。为了便于拆装,测试用的小环天线与光发射机之间选择BNC接口连接。
双环天线应当选择两个尺寸相同的环天线,根据式(2)可知,单环传感器的量程由负载电压VL和环天线的等效面积Ae的范围共同决定。其中,负载电压VL主要由光发射机的输入电压等级决定;等效面积Ae则主要与环天线半径r有关,在进行初步估算时,可以近似认为Ae=πr2。
同时,双环天线之间应保证镜像对称放置条件且保持适宜的环间距L。其中镜像对称是差分计算的基本前提,而环间距是影响环间互扰的主要因素之一。当L过小时,双环天线互扰加剧;当L过大时,两个环天线所处的磁场环境不同,差分后引入误差。因此,应当在互扰程度可接受的前提下取较小的环间距。
针对选定的天线尺寸,为了匹配合适的环间距,本文利用CST Studio进行仿真计算,其仿真设置如图4所示。首先,利用一个环天线作为标准环,测得标准环在1.2/50 μs双指数标准雷电波下的响应波形,记为标准波形;其次,在相同场环境中于相距标准环距离L处增加一个镜像放置的干扰环后,测得标准环上的响应波形,记为干扰波形。
为了评估环间距对互扰程度的影响,本文提出一种综合失真率ε,用以表征双环天线的互扰程度大小,其计算公式给出如下
ε = εu+εt (5) 式中:εu为幅值失真率,用以表征测量波形相较于标准波形在幅值上的失真程度;εt为时间失真率,用以表征测量波形相较于标准波形在上升时间和半宽时间上的失真程度。
εt=|T1i−T1s|/2T1d+|T2i−T2s|/2T2d (6) εu=|Ui−Us|/Ud (7) 式中:Us,Ui分别为标准波形和干扰波形的幅值;Ud表示标准波形与干扰波形之间的幅值差的绝对值;T1s,T1i分别为标准波形和干扰波形的上升时间;T1d表示标准波形与干扰波形之间的上升时间差的绝对值;T2s,T2i分别为标准波形和干扰波形的半宽时间;T2d表示标准波形与干扰波形之间的半宽时间差的绝对值。
互扰仿真时取环直径为10 cm,共设置五组不同的环间距L,分别为1,3,5,7,9 cm。仿真结果见表1。由表1可以看到,当环间距小于5 cm时,综合失真率ε大于5 %。综合考虑,当r=10 cm时,取L=5 cm。最后,根据确定的环半径和环间距,制成固定模具,用于保持双环天线相对位置固定。
表 1 仿真结果Table 1. Results of simulationL/cm εu/% εt/% ε/% 1 10.52 2.58 13.11 3 4.63 2.20 6.83 5 3.27 1.77 4.45 7 0.78 1.51 2.29 9 0.63 1.44 2.07 2.2 光传输系统
脉冲磁场的测量通常伴随着复杂的变化的电磁环境,为了避免传输过程中的干扰,目前常用的是基于光电隔离技术的光传输解决方案[11-13]。本文采用的光传输系统主要由光发射机、单模光纤、光接收机组成。
光发射机用于将输入的磁场微分电信号转换为输出的差分磁场光信号,在其内部依次完成输入信号的积分、差分、放大、非线性补偿、滤噪、电光转换,最终实现光信号的输出。
光发射机内置两块8.4 V锂电池,可以实现±8.4 V直流供能。另外,光发射机采用铝制外壳,以保护信号在其内部传递不受干扰,同时严格限制孔缝结构,使其可以在强电磁场中工作。经试验验证,其屏蔽效能超过80 dB。
单模光纤用于连接光发射机和光接收机,其长度可以根据所需布线长度而调整。通常利用光纤衰减系数来评估光纤对光信号功率的衰减值。对于单模光纤,光纤衰减系数通常取0.18 dB/km[14]。
光接收机通常置于屏蔽良好的室内或者屏蔽柜工作,其输入端通过ST接口与光纤相连,输出端可以通过BNC接口与示波器等采集设备相连。光接收机用于实现磁场光信号到磁场电信号的转换,光信号在其内部经由光电转换、放大、补偿、阻抗转换模块,最终在其输出端复现出光发射机输入级的电信号。
3. 试验验证与结果分析
本文中,我们提出了双环差分测量原理,并设计研制了一种用于近场测量的脉冲磁场传感器,这种新型的磁场传感器,相较于传统的单环传感器具有较强的抗干扰能力,特别是在强电场环境中,能够实现对磁场的准确测量。同时,双环差分结构可以适用于多种环天线,从而匹配不同磁场环境的测量要求,因此在脉冲磁场测量领域有着较为广泛的应用前景。
依据国军标GJB1389A,在实验室搭建了一套邻近雷击环境模拟装置[15],如图5所示。模拟装置主要由Marx发生器,场照射器,截断装置和电容分压器组成。其中,Marx发生器用于产生1.2/50 μs标准雷电波冲击高压;场照射器用于承载被测物并产生相对均匀的电磁脉冲环境;截断装置用于产生截断脉冲高压,形成陡峭的下降沿;电容分压器用于接地系统的高压测量[16]。
由于场照射器采用平行板式,因此在正负电极两端存在巨大瞬时电位差,导致在垂直方向存在强大的瞬变电场。在利用传统单环磁场传感器测量水平磁场时,即使采取较好的屏蔽手段也极易出现测量结果被干扰的现象,因此考虑采用双环差分结构去除电场响应分量,从而实现磁场的准确测量。
对比图6三个波形可以看到:(1) 单环测量波形在0.4~1.5 μs之间存在着强烈的干扰,与双环测量波形在波形前端并不吻合;(2) 单环测量波形前端的干扰在1.5 μs之后消失,与双环测量波形在波形后端基本吻合;(3) CST仿真波形前端与双环天线相似且并不存在振荡分量;(4) 三个波形在波形后端具有很高的一致性,基本符合被测磁场环境。
综上所述,单环测量波形在0.4~1.5 μs之间的波形畸变显然不是磁场响应而应该是电场干扰,可能是由于截断装置击穿空气间隙时产生的高能快速变化的电场导致。对比单环测量和双环测量结果可以看到,双环测量波形通过差分去除了这部分干扰分量从而得到了较为纯净的磁场响应。因此,试验和仿真结果可以证明,双环传感器相较于传统的单环传感器具有更强的抗干扰能力。
4. 结 论
本文提出了双环差分测量原理,并设计研制了一种用于近场测量的脉冲磁场传感器,这种新型的磁场传感器,相较于传统的单环传感器具有较强的抗干扰能力,特别是在强电场环境中,能够实现对磁场的准确测量。同时,双环差分结构可以适用于多种的环天线,从而匹配不同磁场环境的测量要求,因此在脉冲磁场测量领域有着较为广泛的应用前景。
-
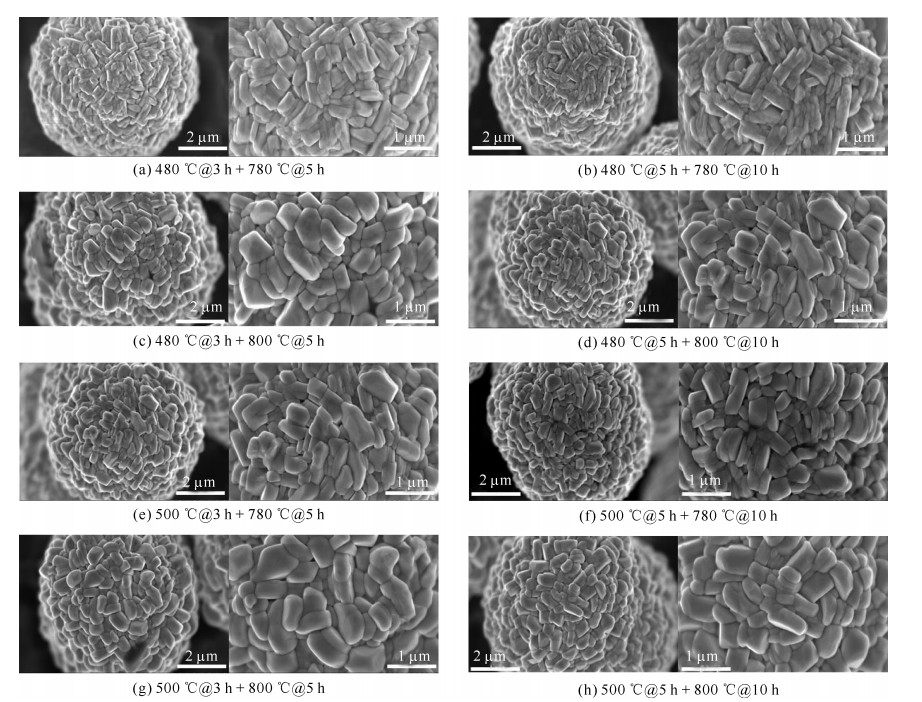
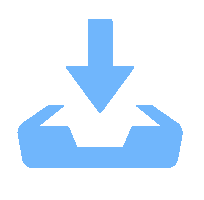
表 1 正极材料LiNi0.8Co0.15Mn0.05O2的烧结工艺
Table 1. Sintering process of cathode material LiNi0.8Co0.15Mn0.05O2
No presintering temperature/℃ presintering time/h calcination temperature/℃ calcination time/h remarks 1 20→480(120 min) 3 480→780 (60 min) 5 cooling 2 20→480(120 min) 5 480→780(60 min) 10 cooling 3 20→480(120 min) 3 480→800(64 min) 5 cooling 4 20→480(120 min) 5 480→800(64 min) 10 cooling 5 20→500(124 min) 3 500→780(56 min) 5 cooling 6 20→500(124 min) 5 500→780(56 min) 10 cooling 7 20→500(124 min) 3 500→800(60 min) 5 cooling 8 20→500(124 min) 5 500→800(60 min) 10 cooling 表 2 不同烧结工艺下正极材料的NCM晶格参数
Table 2. NCM lattice parameters of cathode material under different sintering processes
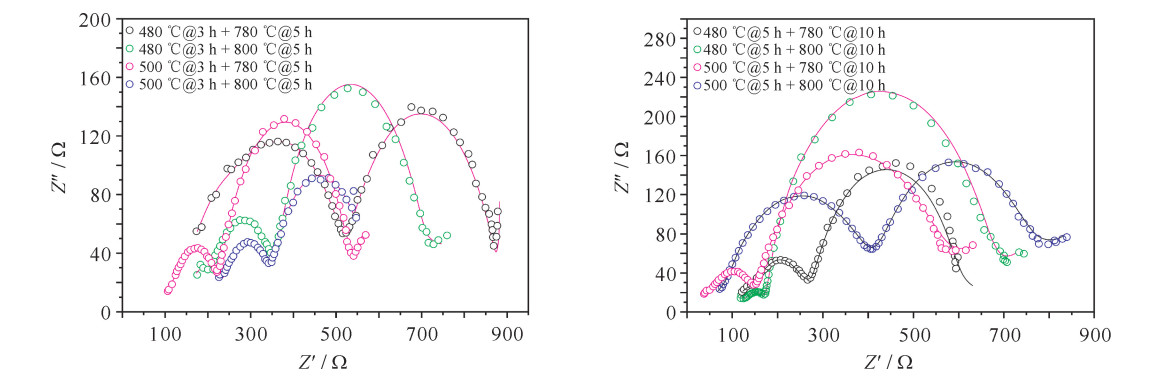
sample a/nm c/nm c/a I(003) I(104) R=I(003)/I(104) 480 ℃@3 h+780 ℃@5 h 0.250 74 1.409 47 5.621 2 100 54.2 1.845 0 480 ℃@3 h+800 ℃@5 h 0.250 48 1.410 10 5.629 6 100 51.0 1.960 8 500℃@3 h+780 ℃@5 h 0.286 45 1.410 94 4.925 6 100 57.4 1.742 2 500℃@3 h+800 ℃@5 h 0.286 38 1.407 10 4.913 4 100 58.9 1.697 8 480 ℃@5 h+780 ℃@10 h 0.250 39 1.410 24 5.632 2 100 54.9 1.821 5 480 ℃@5 h+800 ℃@10 h 0.250 56 1.409 18 5.624 1 100 63.1 1.584 8 500℃@5 h+780 ℃@10 h 0.286 20 1.407 21 4.916 9 100 47.6 2.100 8 500℃@5 h+800 ℃@10 h 0.286 37 1.411 11 4.927 6 100 57.5 1.739 1 表 3 不同烧结工艺下正极材料EIS拟合结果
Table 3. EIS fitting results of cathode material under different sintering processes
sample electrolyte resistivity/Ω RSEI/Ω resistency/Ω Rct/Ω 480 ℃@3 h+780 ℃@5 h 135.40 171.70 211.50 366.20 480 ℃@3 h+800 ℃@5 h 153.50 310.60 146.60 44.84 500℃@3 h+780 ℃@5 h 90.93 43.19 100.30 293.60 500℃@3 h+800 ℃@5 h 0.001 253 237.40 218.50 111.10 480 ℃@5 h+780 ℃@10 h 3.933 271.00 86.11 20.65 480 ℃@5 h+800 ℃@10 h 0.544 1 31.76 134.50 460.50 500℃@5 h+780 ℃@10 h 17.00 36.86 360.60 80.89 500℃@5 h+800 ℃@10 h 27.21 312.7 47.51 276.70 表 4 各材料CV曲线对应的主要氧化还原峰电位及电位差
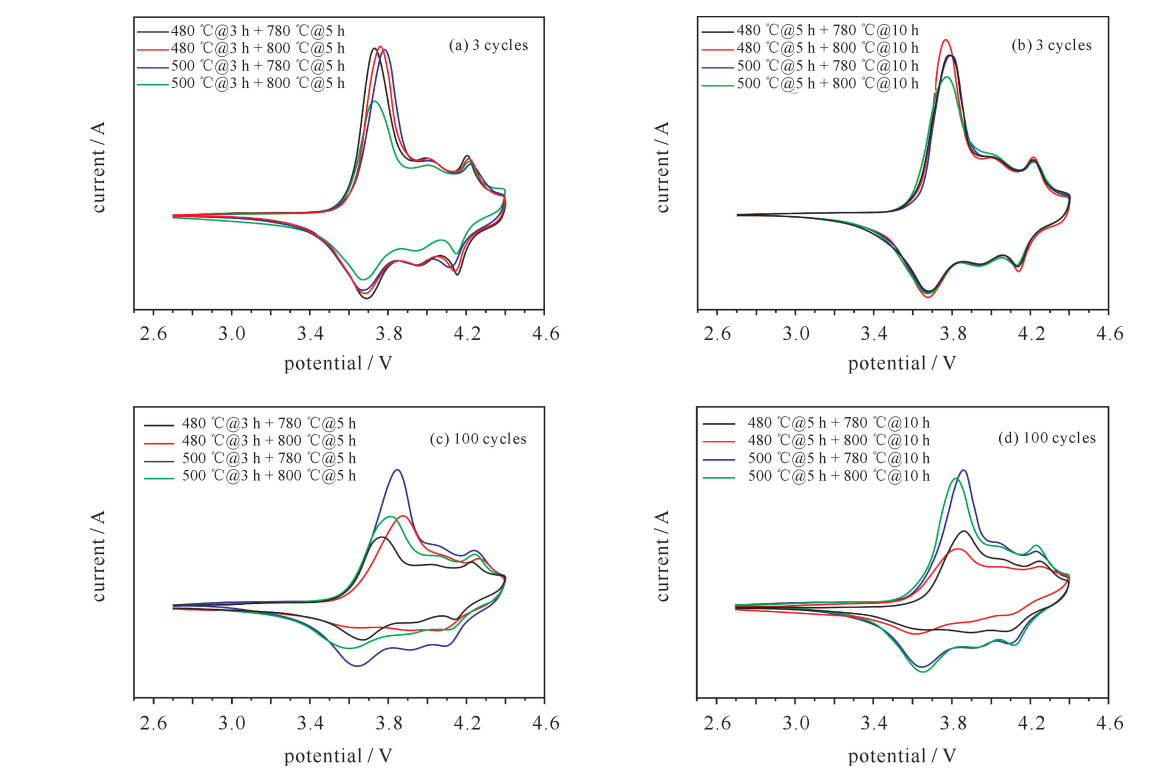
Table 4. Main REDOX peak potential and PD corresponding to CV curve of each material
sample oxidation peak potential/V reduction peak potential/V potential(oxidation peak-reduction peak)/V 3 cycles 100 cycles 3 cycles 100 cycles 3 cycles 100 cycles 480 ℃@3 h+780 ℃@5 h 3.733 3.767 3.699 3.669 0.034 0.098 480 ℃@3 h+800 ℃@5 h 3.760 3.872 3.682 3.628 0.078 0.244 500℃@3 h+780 ℃@5 h 3.784 3.846 3.670 3.639 0.114 0.207 500℃@3 h+800 ℃@5 h 3.732 3.817 3.667 3.592 0.065 0.225 480 ℃@5 h+780 ℃@10 h 3.776 3.859 3.681 3.653 0.095 0.206 480 ℃@5 h+800 ℃@10 h 3.769 3.828 3.680 3.626 0.089 0.202 500℃@5 h+780 ℃@10 h 3.794 3.859 3.679 3.644 0.115 0.215 500℃@5 h+800 ℃@10 h 3.780 3.823 3.680 3.652 0.100 0.171 表 5 三元正极材料电化学性能对比表
Table 5. Properties comparison of ternary layered oxide cathode materials
ternary anode material initial charge-discharge specific capacity at 0.1C magnification/(mA·h·g-1) capacity retention ratio after 100 cycles at 1C/% ref. LiNi0.6Co0.2Mn0.2O2 173.0 89.7 [23] LiNi0.5Co0.2Mn0.3O2 167.4 89.7 [24] LiNi1/3Co1/3Mn1/3O2 152.3 85.8 [25] LiNi0.4Co0.2Mn0.4O2 180.7 90.3 [26] LiNi1-x-yCoxAlyO2 172.7 92.1 [27] LiNi0.8Co0.15Mn0.05O2 200.0 94.0 -
[1] 闫金定. 锂离子电池发展现状及其前景分析[J]. 航空学报, 2014, 35(10): 2767-2775. https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htmYan Jinding. Current status and development analysis of lithium-ion batteries. Acta Aeronautica et Astronautica Sinica, 2014, 35(10): 2767-2775 https://www.cnki.com.cn/Article/CJFDTOTAL-HKXB201410008.htm [2] Li Yangxing, Wan Chunrong, Wu Yuping, et al. Synthesis and characterization of ultrafine LiCoO2 powders by a spray-drying method[J]. Journal of Power Sources, 2000, 85(2): 294-298. doi: 10.1016/S0378-7753(99)00159-7 [3] Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451: 652-657. doi: 10.1038/451652a [4] 庞春会, 吴川, 吴锋, 等. 锂离子电池纳米正极材料合成方法研究进展[J]. 硅酸盐学报, 2012, 40(2): 247-255. doi: 10.14062/j.issn.0454-5648.2012.02.012Pang Chunhui, Wu Chuan, Wu Feng, et al. Development on synthesis methods for nano-materials of cathode for lithium ion battery. Journal of the Chinese Ceramic Society, 2012, 40(2): 247-255 doi: 10.14062/j.issn.0454-5648.2012.02.012 [5] Patil A, Patil V, Shin D W, et al. Issue and challenges facing rechargeable thin film lithium batteries[J]. Mater Res Bull, 2008, 43: 1913-1942. doi: 10.1016/j.materresbull.2007.08.031 [6] Shukla A K, Kumar T P. Materials for next-generation lithium batteries[J]. Curr Sci, 2008, 94: 314-331. [7] Hassoun J, Kim J H, Lee D J. A contribution to the progress of high energy batteries: A metal-free, lithium-ion, silicon-sulfur battery[J]. Journal of Power Sources, 2012, 202: 308-313. doi: 10.1016/j.jpowsour.2011.11.060 [8] 邹邦坤, 丁楚雄, 陈春华. 锂离子电池三元正极材料的研究进展[J]. 中国科学: 化学, 2014, 44(7): 1104-1115. https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htmZou Bangkun, Ding Chuxiong, Chen Chunhua. Research progress in ternary cathode materials Li(Ni, Co, Mn)O2 for lithium ion batteries. Scientia Sinica Chimica, 2014, 44(7): 1104-1115 https://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201407005.htm [9] 黄可龙, 王兆翔, 刘素琴. 锂离子电池原理与关键技术[M]. 北京: 化学工业出版社, 2008: 1-10.Huang Kelong, Wang Zhaoxiang, Liu Suqin. Principle and key technology of lithium ion battery. Beijing: Chemical Industry Press, 2008: 1-10 [10] 马璨, 吕迎春, 李泓. 锂离子电池基础科学问题(Ⅶ)—正极材料[J]. 储能科学与技术, 2014, 3(1): 53-65. doi: 10.3969/j.issn.2095-4239.2014.01.008Ma Can, Lü Yingchun, Li Hong. Fundamental scientific aspects of lithium batteries (Ⅶ)—positive electrode materials. Energy Storage Science and Technology, 2014, 3(1): 53-65 doi: 10.3969/j.issn.2095-4239.2014.01.008 [11] 章福平, 纪勇, 李安东, 等. 锂离子电池正极材料研究的新动向和挑战[J]. 化学通报, 2011, 74(10): 891. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htmZhang Fuping, Ji Yong, Li Andong, et al. Progresses and challenges in studies on cathodic materials of Li-ion battery. Chemistry, 2011, 74(10): 891 https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB201110005.htm [12] Wang Zhao. Studies on Li-excess Mn-based Li1. 2Mn0.54Ni0.13Co0.13O2 as cathode materials for lithium batteries. Beijing: Beijing Institute of Technology, 2015: 1-189 [13] 周燕芳, 钟辉. 锂离子电池正极材料的研究进展[J]. 材料开发与应用, 2003, 18(2): 34-38. doi: 10.3969/j.issn.1003-1545.2003.02.012Zhou Yanfang, Zhong Hui. Research progress in positive electrode materials for lithium ion battery. Development and Application of Materials, 2003, 18(2): 34-38 doi: 10.3969/j.issn.1003-1545.2003.02.012 [14] Muto S S, Tatsumi K S, Kojima Y J, et al. Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods[J]. Power Sources, 2012, 205: 449-455. doi: 10.1016/j.jpowsour.2012.01.071 [15] Cui Y L, Bao W J, Yuan Z, et al. Comparison of different soft chemical routes synthesis of submicro-LiMn2O4 and their influence on its electrochemical properties[J]. Solid State Electrochemistry, 2012, 16(4): 1551-1559. doi: 10.1007/s10008-011-1558-6 [16] 周文彩, 李金洪, 姜晓谦. 磷酸铁锂制备工艺及研究进展[J]. 硅酸盐通报, 2010, 29(1): 134-146. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htmZhou Wencai, Li Jinhong, Jiang Xiaoqian. Preparation and research progress of LiFePO4 cathode material. Bulletin of the Chinese Ceramic Society, 2010, 29(1): 134-146 https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201001031.htm [17] 张新龙, 胡国荣, 彭忠东, 等. 锂离子电池正极材料LiFePO4的研究进展[J]. 电池, 2003, 33(4): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htmZhang Xinlong, Hu Guo-rong, Peng Zhongdong, et al. Development of LiFePO4 as cathode material of Li-ion battery. Battery Bimonthly, 2003, 33(4): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI200304020.htm [18] 沙鸥, 赵敏寿, 翟静, 等. 锂离子电池新型正极材料LiFePO4的研究进展[J]. 稀有金属材料与工程, 2009, 38(11): 252-254. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htmSha Ou, Zhao Minshou, Zhai Jing, et al. Research progress on LiFePO4—A new type of cathode materials for lithium-ion battery. Rare Metal Materials and Engineering, 2009, 38(11): 252-254 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE200911043.htm [19] 翟静, 赵敏寿, 沙鸥, 等. 锂离子电池正极材料Li3V2(PO4)3的研究进展[J]. 稀有金属材料与工程, 2010, 39(7): 1311-1316. https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htmZhai Jing, Zhao Minshou, Sha Ou, et al. Research progress in cathode material Li3V2(PO4)3 for Li-ion batteries. Rare Metal Materials and Engineering, 2010, 39(7): 1311-1316 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE201007040.htm [20] Li Y J, Hong L, Wu F. Preparation of Li3V2(PO4)3 cathode material for power Li-ion batteries[J]. Progress in Chemistry, 2012, 24(1): 47-53. [21] 李龙. 锂离子电池镍钴锰三元正极材料的合成与改性研究[D]. 北京: 清华大学, 2012: 1-54.Li Long. Synthesis and modification of LiNixCoyMnzO2 cathode material for lithium battery. Beijing: Tsinghua University, 2012: 1-54 [22] Ruan Zewen. Synthesis and modification of LiNi1-x-yCoxAlyO2 nickel rich cathode materials. Harbin: Harbin Institute of Technology, 2016: 1-81 [23] 艾灵, 毛丽萍, 李世友, 等. 三元正极材料LiNi0.6Co0.2Mn0.2O2制备及改性方法进展[J]. 化工新型材料, 2018, 46(5): 11-15. https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htmAi Ling, Mao Liping, Li Shiyou, et al. Progress on the preparation and modification of LiNi0.6Co0.2Mn0.2O2 ternary anode material. New Chemical Materials, 2018, 46(5): 11-15 https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201805003.htm [24] 李尚津. 锂离子电池三元正极材料LiNi0.5Co0.2Mn0.3O2的制备与改性[D]. 南宁: 广西大学: 2017: 1-47.Li Shangjin. Preparation and modification of LiNi0.5Co0.2Mn0.3O2 of the lithium ion battery. Nanning: Guangxi University, 2017: 1-47 [25] 路乃群, 王存国, 王静强, 等. 锂离子电池三元正极材料LiNi1/3Co1/3Mn1/3O2的制备方法及其改性研究进展[J]. 化工科技, 2018, 26(6): 58-63. https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htmLu Naiqun, Wang Cunguo, Wang Jingqiang, et al. Development of the ternary cathode material LiNi1/3Co1/3Mn1/3O2 and its modification for lithium ion batteries. Science & Technology in Chemical Industry, 2018, 26(6): 58-63 https://www.cnki.com.cn/Article/CJFDTOTAL-JKGH201806013.htm [26] Wolff-Goodrich S, Lin F, Markus I M, et al. Tailoring the surface properties of LiNi0.4Mn0.4Co0.2O2 by titanium substitution for improved high voltage cycling performance[J]. Physical Chemistry Chemical Physics, 2015, 17(34): 21778-21781. [27] 王义飞, 武行兵, 王双双, 等. 三元正极材料LiNi1- x- yCoxAlyO的研究进展[J]. 电池, 2017, 47(2): 112-115. https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htmWang Yifei, Wu Xingbing, Wang Shuangshuang, et al. Research progress in ternary cathode materials LiNi1-x-yCoxAlyO2. Battery Bimonthly, 2017, 47(2): 112-115 https://www.cnki.com.cn/Article/CJFDTOTAL-DACI201702017.htm -






 下载:
下载:













 下载:
下载: